What is Global High Power Safety Medical Connector Market?
The Global High Power Safety Medical Connector Market refers to the industry that focuses on the production and distribution of connectors specifically designed for high-power applications in medical devices. These connectors are crucial for ensuring the safe and reliable transmission of electrical power and signals in various medical equipment, such as imaging systems, patient monitoring devices, and surgical instruments. The market encompasses a wide range of products, including single-pin connectors, multi-pin connectors, variable connectors, and modular high voltage connectors. These connectors are designed to meet stringent safety standards and regulatory requirements, ensuring that they can withstand high voltages and currents without compromising patient safety or device performance. The market is driven by the increasing demand for advanced medical technologies, the growing prevalence of chronic diseases, and the need for reliable and efficient power solutions in healthcare settings. As medical devices become more sophisticated and require higher power levels, the demand for high power safety medical connectors is expected to continue to grow.
Single-pin Connector, Multi-pin Connector, Variable Connector, Modular High Voltage Connector in the Global High Power Safety Medical Connector Market:
Single-pin connectors, multi-pin connectors, variable connectors, and modular high voltage connectors are essential components in the Global High Power Safety Medical Connector Market. Single-pin connectors are typically used in applications where a simple and reliable connection is needed. They are easy to use and provide a secure connection for transmitting power or signals. These connectors are often found in handheld medical devices, such as diagnostic tools and portable monitors. Multi-pin connectors, on the other hand, are designed for more complex applications that require multiple connections within a single connector. They are commonly used in imaging systems, patient monitoring devices, and other equipment that require the transmission of multiple signals or power lines. Multi-pin connectors offer the advantage of reducing the number of individual connections needed, simplifying the overall design and improving reliability. Variable connectors are designed to provide flexibility in connecting different types of devices. They can be adjusted to accommodate different pin configurations and power requirements, making them suitable for a wide range of medical applications. These connectors are often used in research and development settings, where the ability to quickly and easily reconfigure connections is important. Modular high voltage connectors are designed for applications that require the transmission of high voltages and currents. They are typically used in large medical equipment, such as MRI machines and CT scanners, where the safe and reliable transmission of high power is critical. These connectors are designed to meet stringent safety standards and are often equipped with features such as shielding and insulation to protect against electrical hazards. Overall, the different types of connectors in the Global High Power Safety Medical Connector Market play a crucial role in ensuring the safe and reliable operation of medical devices.
Medical, Electronic, Electric Power, Military, Research, Other in the Global High Power Safety Medical Connector Market:
The Global High Power Safety Medical Connector Market finds extensive usage across various sectors, including medical, electronic, electric power, military, research, and other fields. In the medical sector, these connectors are vital for ensuring the safe and efficient operation of a wide range of medical devices. They are used in imaging systems, patient monitoring devices, surgical instruments, and other equipment that require reliable power and signal transmission. The connectors help to ensure that these devices operate safely and effectively, reducing the risk of electrical hazards and improving patient outcomes. In the electronic sector, high power safety medical connectors are used in the design and manufacture of various electronic devices and systems. They provide reliable connections for transmitting power and signals, ensuring the safe and efficient operation of electronic equipment. In the electric power sector, these connectors are used in the design and maintenance of power distribution systems. They help to ensure the safe and reliable transmission of high voltages and currents, reducing the risk of electrical hazards and improving the efficiency of power distribution. In the military sector, high power safety medical connectors are used in various applications, including communication systems, radar systems, and other equipment that require reliable power and signal transmission. These connectors help to ensure the safe and efficient operation of military equipment, improving the reliability and effectiveness of military operations. In the research sector, high power safety medical connectors are used in the design and testing of new medical devices and technologies. They provide reliable connections for transmitting power and signals, ensuring the safe and efficient operation of research equipment. In other fields, high power safety medical connectors are used in various applications that require reliable power and signal transmission. Overall, the Global High Power Safety Medical Connector Market plays a crucial role in ensuring the safe and efficient operation of a wide range of devices and systems across various sectors.
Global High Power Safety Medical Connector Market Outlook:
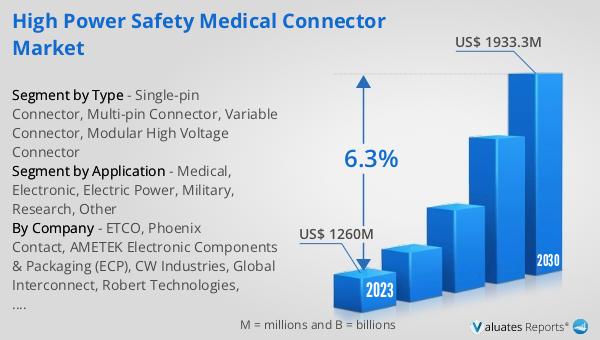
The global High Power Safety Medical Connector market was valued at US$ 1260 million in 2023 and is anticipated to reach US$ 1933.3 million by 2030, witnessing a CAGR of 6.3% during the forecast period 2024-2030. According to our research, the global market for medical devices is estimated at US$ 603 billion in the year 2023 and will be growing at a CAGR of 5% during the next six years. This growth is driven by the increasing demand for advanced medical technologies and the need for reliable and efficient power solutions in healthcare settings. The market for high power safety medical connectors is expected to benefit from these trends, as medical devices become more sophisticated and require higher power levels. The connectors play a crucial role in ensuring the safe and reliable operation of medical devices, reducing the risk of electrical hazards and improving patient outcomes. As the demand for advanced medical technologies continues to grow, the market for high power safety medical connectors is expected to expand, providing opportunities for manufacturers and suppliers in this industry.
| Report Metric | Details |
| Report Name | High Power Safety Medical Connector Market |
| Accounted market size in 2023 | US$ 1260 million |
| Forecasted market size in 2030 | US$ 1933.3 million |
| CAGR | 6.3% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | ETCO, Phoenix Contact, AMETEK Electronic Components & Packaging (ECP), CW Industries, Global Interconnect, Robert Technologies, Da-Green Electronics, LEONI Elocab Ltd, GES High Voltage |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |