What is Global Human Papillomavirus Genotyping Kits Market?
The Global Human Papillomavirus (HPV) Genotyping Kits Market is a specialized segment within the broader medical diagnostics industry, focusing on the detection and identification of various strains of the human papillomavirus. HPV is a significant public health concern due to its association with several types of cancers, including cervical, anal, and oropharyngeal cancers. Genotyping kits are essential tools used by healthcare professionals to determine the specific type of HPV present in a sample, which is crucial for assessing the risk of cancer development and guiding treatment decisions. These kits are designed to be highly sensitive and specific, allowing for accurate detection of both high-risk and low-risk HPV strains. The market for these kits is driven by the increasing prevalence of HPV infections worldwide, growing awareness about the importance of early detection, and advancements in molecular diagnostics technology. Additionally, government initiatives and public health campaigns aimed at reducing the incidence of HPV-related diseases further contribute to the market's growth. As a result, the demand for HPV genotyping kits is expected to continue rising, making it a vital component of the global healthcare landscape.
High Risk, Low Risk in the Global Human Papillomavirus Genotyping Kits Market:
In the Global Human Papillomavirus Genotyping Kits Market, the classification of HPV strains into high-risk and low-risk categories plays a crucial role in understanding the potential health implications of an infection. High-risk HPV types, such as HPV 16 and 18, are known to be the primary culprits behind the majority of HPV-related cancers. These strains have the ability to integrate into the host's DNA, leading to cellular changes that can eventually result in malignancy. The presence of high-risk HPV types in a patient significantly increases the likelihood of developing cervical cancer, among other types. Consequently, genotyping kits that can accurately identify these high-risk strains are indispensable in clinical settings, as they help healthcare providers assess the cancer risk and determine the most appropriate follow-up actions, such as more frequent screenings or preventive treatments like vaccines. On the other hand, low-risk HPV types, such as HPV 6 and 11, are generally associated with benign conditions like genital warts and respiratory papillomatosis. While these strains do not typically lead to cancer, they can cause significant discomfort and require medical intervention to manage symptoms. The ability to differentiate between high-risk and low-risk HPV types is essential for tailoring patient management strategies. For instance, a patient diagnosed with a low-risk HPV type may not require the same level of monitoring or intervention as someone with a high-risk type. This differentiation also aids in reducing unnecessary anxiety and medical procedures for patients with low-risk infections. The market for HPV genotyping kits is influenced by several factors, including the rising incidence of HPV infections globally and the increasing awareness of the importance of early detection and prevention. As more people become aware of the link between HPV and cancer, there is a growing demand for reliable diagnostic tools that can provide accurate information about an individual's HPV status. This demand is further fueled by public health initiatives and vaccination programs aimed at reducing the prevalence of HPV-related diseases. In many countries, HPV vaccination programs have been implemented to protect against the most common high-risk strains, thereby reducing the overall burden of HPV-related cancers. Technological advancements in molecular diagnostics have also played a significant role in shaping the HPV genotyping kits market. Modern genotyping kits are designed to be highly sensitive and specific, allowing for the detection of multiple HPV types in a single test. This capability is particularly important in clinical settings, where timely and accurate results are essential for guiding patient management decisions. Additionally, the development of user-friendly and cost-effective kits has made HPV genotyping more accessible to a broader range of healthcare providers, including those in resource-limited settings. Despite the advancements in HPV genotyping technology, there are still challenges that need to be addressed. One of the primary challenges is the variability in the availability and affordability of these kits across different regions. In low- and middle-income countries, access to advanced diagnostic tools may be limited due to financial constraints and lack of infrastructure. Efforts to improve access to HPV genotyping kits in these regions are crucial for achieving global health equity and reducing the incidence of HPV-related diseases worldwide. In conclusion, the classification of HPV strains into high-risk and low-risk categories is a fundamental aspect of the Global Human Papillomavirus Genotyping Kits Market. Accurate identification of these strains is essential for assessing cancer risk and guiding patient management decisions. The market is driven by the increasing prevalence of HPV infections, growing awareness of the importance of early detection, and advancements in molecular diagnostics technology. However, challenges related to access and affordability remain, highlighting the need for continued efforts to improve the availability of HPV genotyping kits globally.
Medical, Research, Others in the Global Human Papillomavirus Genotyping Kits Market:
The usage of Global Human Papillomavirus Genotyping Kits Market spans several critical areas, including medical, research, and other applications, each playing a vital role in combating HPV-related health issues. In the medical field, these kits are primarily used for screening and diagnosing HPV infections. Healthcare providers rely on genotyping kits to identify the specific strains of HPV present in patients, which is crucial for determining the risk of developing HPV-related cancers. Early detection through genotyping allows for timely intervention, such as increased surveillance, preventive measures, or treatment options, thereby improving patient outcomes. Moreover, genotyping results can guide vaccination decisions, ensuring that individuals receive the most appropriate vaccine to protect against high-risk HPV types. In research settings, HPV genotyping kits are indispensable tools for studying the epidemiology and pathogenesis of HPV infections. Researchers use these kits to investigate the prevalence and distribution of different HPV strains in various populations, which helps in understanding the dynamics of HPV transmission and the factors contributing to the development of HPV-related diseases. This research is essential for informing public health strategies and developing targeted interventions to reduce the burden of HPV infections. Additionally, genotyping kits are used in clinical trials to evaluate the efficacy of new vaccines and therapeutic approaches, contributing to the advancement of HPV-related healthcare. Beyond medical and research applications, HPV genotyping kits have other important uses. For instance, they are employed in public health surveillance programs to monitor the impact of vaccination campaigns and assess the effectiveness of existing prevention strategies. By tracking changes in the prevalence of different HPV strains over time, public health officials can make informed decisions about vaccination policies and resource allocation. Furthermore, genotyping kits are used in educational initiatives aimed at raising awareness about HPV and its associated risks. By providing accurate information about the types of HPV and their potential health implications, these initiatives help to promote preventive behaviors and encourage individuals to participate in screening and vaccination programs. The versatility of HPV genotyping kits in various applications underscores their importance in the global effort to combat HPV-related diseases. As the demand for these kits continues to grow, driven by increasing awareness and technological advancements, it is essential to ensure their accessibility and affordability across different regions. Efforts to expand the availability of genotyping kits, particularly in low- and middle-income countries, are crucial for achieving global health equity and reducing the incidence of HPV-related cancers worldwide. By leveraging the capabilities of HPV genotyping kits in medical, research, and other applications, we can make significant strides in preventing and managing HPV infections, ultimately improving public health outcomes on a global scale.
Global Human Papillomavirus Genotyping Kits Market Outlook:
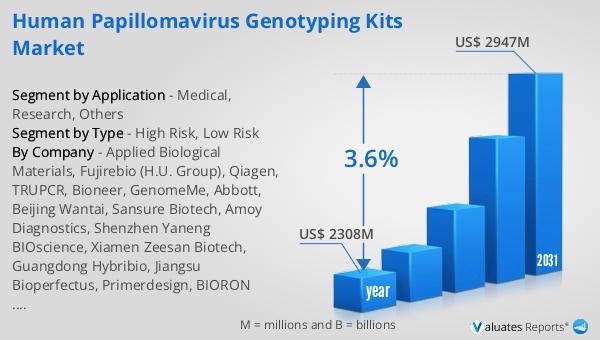
The global market for Human Papillomavirus Genotyping Kits was valued at $2,308 million in 2024 and is anticipated to grow to a revised size of $2,947 million by 2031, reflecting a compound annual growth rate (CAGR) of 3.6% during the forecast period. This growth trajectory underscores the increasing demand for HPV genotyping kits, driven by the rising prevalence of HPV infections and the growing awareness of the importance of early detection and prevention. As healthcare systems worldwide continue to prioritize the management of HPV-related diseases, the need for reliable and accurate diagnostic tools becomes more critical. The projected market growth also highlights the impact of technological advancements in molecular diagnostics, which have made HPV genotyping more accessible and efficient. These advancements have enabled the development of highly sensitive and specific kits that can detect multiple HPV types in a single test, thereby enhancing the ability of healthcare providers to make informed decisions about patient care. Furthermore, the market's expansion reflects the ongoing efforts to improve access to HPV genotyping kits in resource-limited settings, ensuring that individuals in all regions have the opportunity to benefit from early detection and intervention. As the market continues to evolve, it is essential to address challenges related to affordability and availability, particularly in low- and middle-income countries, to achieve global health equity and reduce the burden of HPV-related diseases.
| Report Metric | Details |
| Report Name | Human Papillomavirus Genotyping Kits Market |
| Accounted market size in year | US$ 2308 million |
| Forecasted market size in 2031 | US$ 2947 million |
| CAGR | 3.6% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Applied Biological Materials, Fujirebio (H.U. Group), Qiagen, TRUPCR, Bioneer, GenomeMe, Abbott, Beijing Wantai, Sansure Biotech, Amoy Diagnostics, Shenzhen Yaneng BIOscience, Xiamen Zeesan Biotech, Guangdong Hybribio, Jiangsu Bioperfectus, Primerdesign, BIORON Diagnostics, Jiangsu Mole Bioscience, Ningbo Health Gene Technologies, Shenzhen Yilifang Biotechnology, Guangzhou HEAS Bio Tech, Hangzhou ACON Biotech |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
