What is Global Rifampicin API Market?
The Global Rifampicin API Market is a crucial segment within the pharmaceutical industry, primarily focused on the production and distribution of Rifampicin, an antibiotic used to treat several bacterial infections, including tuberculosis and leprosy. Rifampicin is an active pharmaceutical ingredient (API) that plays a vital role in the formulation of various medications. The market for Rifampicin API is driven by the increasing prevalence of tuberculosis and other bacterial infections worldwide, necessitating effective treatment options. Additionally, the rise in healthcare expenditure and the growing awareness about infectious diseases contribute to the market's expansion. The demand for Rifampicin API is also influenced by the need for high-quality, cost-effective drugs in developing countries, where the burden of infectious diseases is often higher. As a result, pharmaceutical companies are investing in research and development to enhance the production processes and improve the efficacy of Rifampicin-based medications. The market is characterized by a competitive landscape, with numerous manufacturers striving to meet the global demand while adhering to stringent regulatory standards. Overall, the Global Rifampicin API Market is poised for growth, driven by the ongoing need for effective treatments against bacterial infections.
Above 98 %, Above 99 % in the Global Rifampicin API Market:
In the Global Rifampicin API Market, the purity levels of the active pharmaceutical ingredient are critical, with common specifications being "Above 98%" and "Above 99%." These purity levels are essential for ensuring the efficacy and safety of the medications produced. The "Above 98%" purity level indicates that the Rifampicin API contains more than 98% of the active ingredient, with the remaining percentage comprising impurities or other substances. This level of purity is generally considered acceptable for many pharmaceutical applications, providing a balance between cost and quality. However, for certain applications where higher precision and efficacy are required, the "Above 99%" purity level is preferred. This higher purity level ensures that the API contains minimal impurities, which can be crucial for sensitive formulations or for patients with specific health conditions. The choice between these purity levels depends on various factors, including the intended use of the medication, regulatory requirements, and cost considerations. Pharmaceutical companies must carefully evaluate these factors to determine the appropriate purity level for their products. The production of Rifampicin API with these high purity levels involves advanced manufacturing processes and stringent quality control measures. Manufacturers must adhere to Good Manufacturing Practices (GMP) and comply with international regulatory standards to ensure the safety and efficacy of their products. This includes rigorous testing and validation procedures to verify the purity and quality of the API. The demand for high-purity Rifampicin API is driven by the need for effective treatments for tuberculosis and other bacterial infections, particularly in regions with high disease prevalence. As a result, manufacturers are continually investing in research and development to improve production processes and enhance the quality of their products. The competitive landscape of the Global Rifampicin API Market is characterized by numerous players striving to meet the growing demand for high-purity APIs. Companies are focusing on innovation and technological advancements to gain a competitive edge and expand their market share. Additionally, strategic partnerships and collaborations are common in this market, enabling companies to leverage each other's strengths and resources. The market is also influenced by regulatory developments, with authorities imposing strict guidelines to ensure the safety and efficacy of pharmaceutical products. Compliance with these regulations is essential for manufacturers to maintain their market position and reputation. Overall, the Global Rifampicin API Market is a dynamic and competitive industry, driven by the need for high-quality, effective treatments for bacterial infections. The focus on purity levels, particularly "Above 98%" and "Above 99%," underscores the importance of quality and safety in the production of Rifampicin API. As the demand for effective treatments continues to rise, manufacturers are poised to play a crucial role in addressing global health challenges.
Tablets, Capsules, Suspension, Others in the Global Rifampicin API Market:
The Global Rifampicin API Market finds its application in various pharmaceutical formulations, including tablets, capsules, suspensions, and others. Tablets are one of the most common forms of medication, offering convenience and ease of administration. Rifampicin tablets are widely used in the treatment of tuberculosis and other bacterial infections, providing a precise dosage of the active ingredient. The formulation of tablets involves compressing the Rifampicin API with other excipients to create a solid, stable product that can be easily ingested. Capsules, on the other hand, offer an alternative to tablets, particularly for patients who may have difficulty swallowing solid forms of medication. Rifampicin capsules contain the API in a powdered or granular form, enclosed within a gelatin shell. This form allows for the rapid release of the active ingredient in the digestive tract, ensuring quick absorption and efficacy. Capsules are often preferred for their ease of use and the ability to mask the taste of the medication. Suspensions are liquid formulations that contain the Rifampicin API in a finely divided form, suspended in a liquid medium. This form is particularly useful for pediatric and geriatric patients who may have difficulty swallowing tablets or capsules. Suspensions allow for flexible dosing and can be easily administered with a measuring spoon or syringe. The formulation of suspensions requires careful consideration of the stability and solubility of the API to ensure consistent dosing and efficacy. In addition to these common forms, the Global Rifampicin API Market also includes other formulations, such as injectables and topical preparations. Injectable forms of Rifampicin are used in cases where rapid onset of action is required, or when oral administration is not feasible. These formulations require sterile manufacturing processes and strict quality control measures to ensure safety and efficacy. Topical preparations, such as creams and ointments, are used for localized infections and require the Rifampicin API to be formulated in a way that allows for effective penetration of the skin barrier. The choice of formulation depends on various factors, including the nature of the infection, patient preferences, and clinical considerations. Pharmaceutical companies must carefully evaluate these factors to determine the most appropriate form of medication for their target market. The versatility of Rifampicin API in various formulations underscores its importance in the treatment of bacterial infections and highlights the need for continued innovation and development in this field.
Global Rifampicin API Market Outlook:
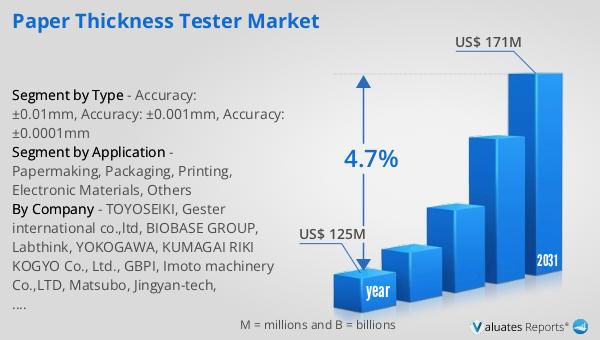
The global pharmaceutical market was valued at approximately 1,475 billion USD in 2022, with an anticipated compound annual growth rate (CAGR) of 5% over the next six years. This growth trajectory reflects the increasing demand for pharmaceutical products driven by factors such as rising healthcare expenditure, an aging population, and the prevalence of chronic diseases. In comparison, the chemical drug market has also shown significant growth, expanding from 1,005 billion USD in 2018 to an estimated 1,094 billion USD in 2022. This growth in the chemical drug sector highlights the ongoing demand for traditional pharmaceutical products, even as the industry experiences shifts towards biologics and personalized medicine. The chemical drug market's expansion is supported by advancements in drug development and manufacturing processes, enabling the production of high-quality, cost-effective medications. As the pharmaceutical industry continues to evolve, both the global market and the chemical drug sector are poised to play a crucial role in addressing global health challenges and meeting the needs of patients worldwide. The sustained growth in these markets underscores the importance of innovation, research, and development in driving the future of healthcare.
| Report Metric | Details |
| Report Name | Rifampicin API Market |
| CAGR | 5% |
| by Type |
|
| by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | EUROAPI, Otto Brandes, CKD Bio Corporation, Arudavis Labs |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |