What is Global Tigecycline API Market?
The Global Tigecycline API Market refers to the worldwide industry focused on the production and distribution of the active pharmaceutical ingredient (API) used in tigecycline, an antibiotic primarily used to treat complicated infections. Tigecycline is a glycylcycline antibiotic that is effective against a wide range of bacteria, including those resistant to other antibiotics. The market for Tigecycline API is driven by the increasing prevalence of antibiotic-resistant infections, which necessitates the development and use of more potent antibiotics like tigecycline. The demand for Tigecycline API is also influenced by the growing healthcare needs of an aging global population, as well as the expansion of healthcare infrastructure in emerging markets. Additionally, the market is shaped by regulatory policies, technological advancements in pharmaceutical manufacturing, and the competitive landscape among pharmaceutical companies. As healthcare providers and patients seek effective treatments for complex infections, the Global Tigecycline API Market plays a crucial role in ensuring the availability and accessibility of this important antibiotic. The market's growth is supported by ongoing research and development efforts aimed at improving the efficacy and safety of tigecycline, as well as exploring new therapeutic applications.
Above 98 %, Above 99 % in the Global Tigecycline API Market:
In the Global Tigecycline API Market, the purity levels of the API are critical factors that influence its effectiveness and safety. Two common purity levels are "Above 98%" and "Above 99%," which refer to the percentage of the API that is pure tigecycline, with minimal impurities. The distinction between these purity levels is significant, as higher purity levels generally indicate a more refined and potent product. APIs with a purity level of "Above 98%" are considered high-quality and suitable for most pharmaceutical applications. However, APIs with a purity level of "Above 99%" are often preferred for more sensitive applications, where even minor impurities could impact the drug's performance or safety. The production of high-purity Tigecycline API requires advanced manufacturing processes and stringent quality control measures to ensure that impurities are minimized. This involves sophisticated techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry to accurately measure and control the purity levels. The choice between "Above 98%" and "Above 99%" purity levels depends on various factors, including the intended use of the API, regulatory requirements, and cost considerations. Pharmaceutical companies must balance the need for high purity with the economic feasibility of production, as achieving higher purity levels can increase manufacturing costs. Additionally, regulatory agencies may have specific guidelines regarding the acceptable purity levels for APIs used in different types of medications. In the context of tigecycline, achieving high purity is particularly important due to its use in treating serious infections, where the margin for error is minimal. Impurities in the API could potentially lead to adverse reactions or reduced efficacy, which is why pharmaceutical manufacturers invest heavily in quality assurance processes. The demand for high-purity Tigecycline API is also driven by the increasing prevalence of antibiotic-resistant bacteria, which necessitates the use of highly effective antibiotics. As a result, pharmaceutical companies are continuously working to improve their manufacturing processes to achieve higher purity levels while maintaining cost-effectiveness. The competitive landscape of the Global Tigecycline API Market is characterized by the presence of several key players, each striving to offer high-quality products that meet the stringent requirements of healthcare providers and regulatory agencies. These companies invest in research and development to enhance their production capabilities and ensure that their APIs meet the highest standards of purity and efficacy. The market is also influenced by technological advancements, such as the development of new purification techniques and the use of advanced analytical tools to monitor and control the purity of the API. As the demand for tigecycline continues to grow, driven by the need for effective treatments for complex infections, the importance of high-purity APIs in the Global Tigecycline API Market cannot be overstated. Pharmaceutical companies must navigate the challenges of producing high-purity APIs while adhering to regulatory requirements and managing production costs. The ongoing efforts to improve the purity and quality of Tigecycline API are essential to ensuring the availability of safe and effective antibiotics for patients worldwide.
Injection, Others in the Global Tigecycline API Market:
The Global Tigecycline API Market plays a vital role in the pharmaceutical industry, particularly in the production of injectable antibiotics. Tigecycline is primarily administered through injection, making it a crucial component in the treatment of severe bacterial infections. The injectable form of tigecycline is used in hospitals and healthcare settings to treat complicated skin and soft tissue infections, intra-abdominal infections, and community-acquired bacterial pneumonia. The use of tigecycline injections is particularly important in cases where oral antibiotics are ineffective or inappropriate, such as in patients with severe infections or those who are unable to take oral medications. The demand for tigecycline injections is driven by the increasing prevalence of antibiotic-resistant infections, which require potent antibiotics like tigecycline for effective treatment. The Global Tigecycline API Market ensures the availability of high-quality APIs necessary for the production of these injectable antibiotics, which are essential for managing complex infections in healthcare settings. In addition to injections, the Global Tigecycline API Market also supports the development of other formulations and applications of tigecycline. While injections remain the primary mode of administration, research and development efforts are underway to explore alternative delivery methods and formulations that could enhance the drug's efficacy and patient compliance. These efforts include the development of oral formulations, extended-release formulations, and combination therapies that leverage the unique properties of tigecycline. The exploration of new applications for tigecycline is driven by the need to address the growing challenge of antibiotic resistance and to provide healthcare providers with a broader range of treatment options. The Global Tigecycline API Market is instrumental in supporting these efforts by providing the necessary APIs for research and development activities. The market's focus on quality and innovation ensures that pharmaceutical companies have access to high-purity APIs that meet the stringent requirements of regulatory agencies and healthcare providers. As the demand for effective antibiotics continues to rise, the Global Tigecycline API Market remains a critical component of the pharmaceutical industry, supporting the development and production of life-saving medications. The market's emphasis on quality, innovation, and collaboration with healthcare providers and regulatory agencies ensures that tigecycline remains a valuable tool in the fight against antibiotic-resistant infections. By providing high-quality APIs for the production of injectable antibiotics and supporting the exploration of new formulations and applications, the Global Tigecycline API Market plays a crucial role in advancing healthcare and improving patient outcomes worldwide.
Global Tigecycline API Market Outlook:
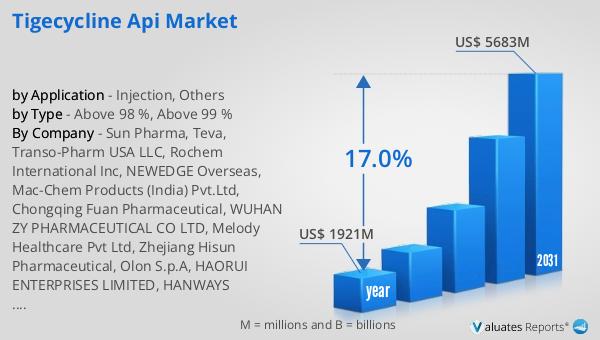
The global market for Tigecycline API was valued at $1,921 million in 2024 and is anticipated to expand to a revised size of $5,683 million by 2031, reflecting a compound annual growth rate (CAGR) of 17.0% during the forecast period. This significant growth underscores the increasing demand for Tigecycline API, driven by the rising prevalence of antibiotic-resistant infections and the need for effective treatments. In contrast, the global pharmaceutical market was valued at $1,475 billion in 2022, with a projected CAGR of 5% over the next six years. This comparison highlights the rapid growth of the Tigecycline API market relative to the broader pharmaceutical industry. Additionally, the chemical drug market, which was estimated at $1,005 billion in 2018, is expected to reach $1,094 billion by 2022. The robust growth of the Tigecycline API market reflects the critical role of this antibiotic in addressing the global challenge of antibiotic resistance. As healthcare providers and patients increasingly rely on tigecycline for the treatment of complex infections, the market's expansion is supported by ongoing research and development efforts, technological advancements, and the commitment of pharmaceutical companies to deliver high-quality APIs. The Global Tigecycline API Market's impressive growth trajectory underscores its importance in the pharmaceutical industry and its potential to drive innovation and improve patient outcomes worldwide.
| Report Metric | Details |
| Report Name | Tigecycline API Market |
| Accounted market size in year | US$ 1921 million |
| Forecasted market size in 2031 | US$ 5683 million |
| CAGR | 17.0% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| by Type |
|
| by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Sun Pharma, Teva, Transo-Pharm USA LLC, Rochem International Inc, NEWEDGE Overseas, Mac-Chem Products (India) Pvt.Ltd, Chongqing Fuan Pharmaceutical, WUHAN ZY PHARMACEUTICAL CO LTD, Melody Healthcare Pvt Ltd, Zhejiang Hisun Pharmaceutical, Olon S.p.A, HAORUI ENTERPRISES LIMITED, HANWAYS PHARMCHEM CO., LTD., Sumar Biotech |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
