What is Global Recombinant Human Serum Albumin Test Market?
The Global Recombinant Human Serum Albumin Test Market is a specialized segment within the broader biotechnology and healthcare industry. Recombinant human serum albumin (rHSA) is a synthetic form of human serum albumin, a protein commonly found in human blood plasma. This protein plays a crucial role in maintaining blood volume and pressure, transporting hormones, and binding drugs. The recombinant version is produced using genetic engineering techniques, making it a valuable tool in various medical and research applications. The market for rHSA tests is driven by the increasing demand for safer and more effective pharmaceutical products, as well as the need for reliable diagnostic tools. These tests are essential for ensuring the quality and efficacy of rHSA used in therapeutic applications, including drug formulation and vaccine production. As the healthcare industry continues to advance, the demand for recombinant human serum albumin tests is expected to grow, driven by the need for precision medicine and personalized healthcare solutions. The market is characterized by ongoing research and development efforts aimed at improving test accuracy and expanding the range of applications for rHSA.
Instruments, Consumables, Others in the Global Recombinant Human Serum Albumin Test Market:
In the Global Recombinant Human Serum Albumin Test Market, the components can be broadly categorized into instruments, consumables, and others. Instruments are the backbone of any testing process, providing the necessary technology and machinery to conduct accurate and efficient tests. These include advanced spectrometers, chromatography systems, and automated analyzers that are designed to handle the specific requirements of recombinant human serum albumin testing. The precision and reliability of these instruments are paramount, as they directly impact the quality of the test results. Manufacturers are continually innovating to enhance the sensitivity and specificity of these instruments, ensuring they meet the stringent standards required in medical and research settings. Consumables, on the other hand, are the materials and reagents used during the testing process. These include test kits, vials, pipettes, and other disposable items that are essential for conducting tests. The quality of consumables is critical, as they must be compatible with the instruments and provide consistent results. The market for consumables is driven by the need for high-quality, reliable products that can support the growing demand for recombinant human serum albumin tests. Companies in this space are focused on developing consumables that are not only effective but also cost-efficient, catering to the needs of both large-scale laboratories and smaller research facilities. The 'others' category encompasses a range of additional components and services that support the testing process. This includes software solutions for data analysis and management, as well as maintenance and calibration services for instruments. The integration of advanced software solutions is becoming increasingly important, as it allows for more efficient data handling and improved accuracy in test results. Additionally, the availability of comprehensive maintenance services ensures that instruments remain in optimal condition, reducing downtime and enhancing productivity. Overall, the Global Recombinant Human Serum Albumin Test Market is a dynamic and evolving space, with a strong focus on innovation and quality. Companies operating in this market are continually seeking ways to improve their offerings, whether through the development of more advanced instruments, the production of higher-quality consumables, or the provision of comprehensive support services. As the demand for recombinant human serum albumin tests continues to grow, driven by advancements in healthcare and biotechnology, the market is poised for significant expansion.
Hospitals and Clinics, Diagnostic Laboratories, Others in the Global Recombinant Human Serum Albumin Test Market:
The usage of the Global Recombinant Human Serum Albumin Test Market spans several key areas, including hospitals and clinics, diagnostic laboratories, and other specialized settings. In hospitals and clinics, recombinant human serum albumin tests are used to ensure the safety and efficacy of treatments involving rHSA. These tests are crucial for monitoring patients who are receiving therapies that include recombinant human serum albumin, as they help healthcare providers assess the protein's performance and detect any potential adverse reactions. The ability to accurately measure and analyze rHSA levels in patients is essential for optimizing treatment plans and improving patient outcomes. In diagnostic laboratories, recombinant human serum albumin tests play a vital role in research and development. These laboratories are often at the forefront of innovation, using rHSA tests to explore new therapeutic applications and improve existing treatments. The precision and reliability of these tests are critical, as they provide the data needed to advance scientific understanding and drive the development of new medical products. Researchers rely on these tests to validate their findings and ensure that their work meets the highest standards of quality and accuracy. Beyond hospitals and diagnostic laboratories, recombinant human serum albumin tests are also used in other specialized settings, such as pharmaceutical companies and academic research institutions. In these environments, the tests are used to support the development and production of new drugs and vaccines. The ability to accurately measure rHSA levels is essential for ensuring the quality and consistency of these products, as well as for meeting regulatory requirements. Pharmaceutical companies, in particular, rely on these tests to ensure that their products are safe and effective for use in humans. Academic research institutions use recombinant human serum albumin tests to explore new scientific questions and contribute to the broader body of knowledge in the field. Overall, the Global Recombinant Human Serum Albumin Test Market is a critical component of the healthcare and biotechnology industries, providing the tools and data needed to advance medical science and improve patient care. As the demand for precision medicine and personalized healthcare solutions continues to grow, the importance of these tests is only expected to increase.
Global Recombinant Human Serum Albumin Test Market Outlook:
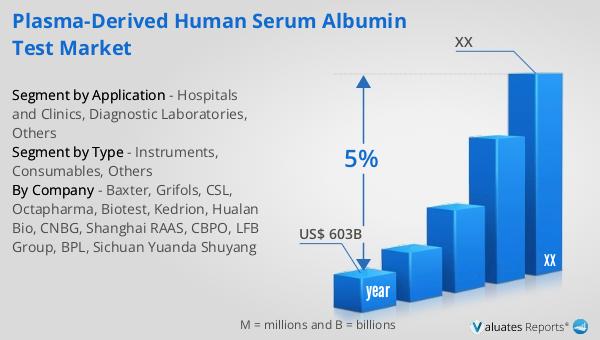
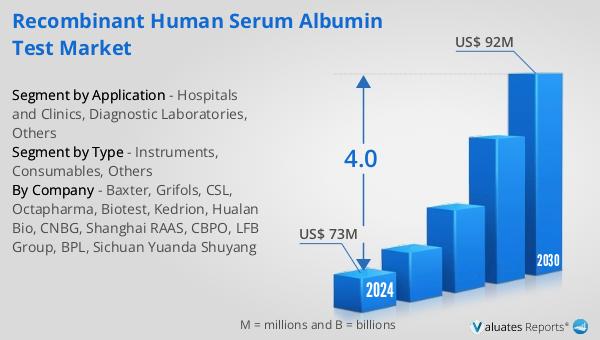
The outlook for the global Recombinant Human Serum Albumin Test market indicates a promising growth trajectory. It is anticipated that the market will expand from $73 million in 2024 to $92 million by 2030, reflecting a compound annual growth rate (CAGR) of 4.0% over the forecast period. This growth is indicative of the increasing demand for recombinant human serum albumin tests, driven by advancements in healthcare and biotechnology. The broader medical devices market, which is estimated to be valued at $603 billion in 2023, is also expected to grow at a CAGR of 5% over the next six years. This growth in the medical devices sector underscores the expanding opportunities for recombinant human serum albumin tests, as they are integral to various diagnostic and therapeutic applications. The increasing focus on precision medicine and personalized healthcare solutions is likely to further drive the demand for these tests, as they provide the necessary data and insights to support these advanced approaches. As the healthcare industry continues to evolve, the global Recombinant Human Serum Albumin Test market is well-positioned to capitalize on these trends, offering innovative solutions that meet the needs of healthcare providers, researchers, and patients alike.
| Report Metric | Details |
| Report Name | Recombinant Human Serum Albumin Test Market |
| Accounted market size in 2024 | US$ 73 million |
| Forecasted market size in 2030 | US$ 92 million |
| CAGR | 4.0 |
| Base Year | 2024 |
| Forecasted years | 2025 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Baxter, Grifols, CSL, Octapharma, Biotest, Kedrion, Hualan Bio, CNBG, Shanghai RAAS, CBPO, LFB Group, BPL, Sichuan Yuanda Shuyang |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |