What is Global Lyme Disease Diagnostics Market?
The Global Lyme Disease Diagnostics Market is a rapidly evolving sector dedicated to the detection and diagnosis of Lyme disease, a tick-borne illness caused by the bacterium Borrelia burgdorferi. This market encompasses a wide range of diagnostic tools and technologies designed to accurately identify the presence of Lyme disease in patients. As awareness of Lyme disease increases and the incidence of the disease rises, the demand for effective diagnostic solutions has grown significantly. The market includes various diagnostic methods such as serological tests, urine antigen tests, and nucleic acid tests, each offering unique advantages in terms of sensitivity, specificity, and speed of results. The development and refinement of these diagnostic tools are crucial for early detection and treatment, which can significantly improve patient outcomes. The market is driven by advancements in technology, increased funding for research, and a growing emphasis on early diagnosis and treatment. As a result, the Global Lyme Disease Diagnostics Market is poised for substantial growth, offering significant opportunities for companies involved in the development and distribution of diagnostic products.
Serological Tests, Urine Antigen Tests, Lymphocytic Transformation Tests, Immunofluorescent Staining, Nucleic Acid Tests in the Global Lyme Disease Diagnostics Market:
Serological tests are a cornerstone of the Global Lyme Disease Diagnostics Market, primarily because they detect antibodies produced by the immune system in response to Borrelia burgdorferi infection. These tests, including enzyme-linked immunosorbent assays (ELISA) and Western blot tests, are widely used due to their ability to provide reliable results. ELISA is often the first step in Lyme disease testing, screening for antibodies in the blood. If ELISA results are positive or equivocal, a Western blot test is typically performed to confirm the diagnosis. This two-tiered approach enhances the accuracy of Lyme disease diagnosis, although it may not detect the disease in its early stages when antibodies have not yet developed. Urine antigen tests, on the other hand, aim to detect the presence of Borrelia antigens in urine samples. These tests can be useful for identifying active infections, but their reliability can vary, and they are not as commonly used as serological tests. Lymphocytic transformation tests (LTT) are another diagnostic method that measures the proliferation of lymphocytes in response to Borrelia antigens. While LTT can provide insights into the immune response, they are complex and not widely available. Immunofluorescent staining is a technique that uses fluorescent dyes to detect Borrelia bacteria in tissue samples. This method can be highly specific but requires specialized equipment and expertise. Nucleic acid tests, such as polymerase chain reaction (PCR), are highly sensitive and can detect the genetic material of Borrelia bacteria. PCR tests are particularly useful for diagnosing Lyme disease in its early stages, as they can identify the presence of the bacteria before antibodies are produced. However, PCR tests can be expensive and require sophisticated laboratory infrastructure. Each of these diagnostic methods plays a vital role in the Global Lyme Disease Diagnostics Market, offering different benefits and limitations. The choice of test often depends on the stage of the disease, the availability of resources, and the clinical presentation of the patient. As research continues to advance, the development of more accurate and accessible diagnostic tools remains a priority, ensuring that patients receive timely and effective care.
Hospitals, Clinics, Others in the Global Lyme Disease Diagnostics Market:
The usage of the Global Lyme Disease Diagnostics Market spans various healthcare settings, including hospitals, clinics, and other medical facilities. In hospitals, the demand for Lyme disease diagnostics is driven by the need for comprehensive testing capabilities to manage a wide range of patient cases. Hospitals often have access to advanced diagnostic technologies and specialized personnel, allowing them to perform a variety of tests, from serological assays to nucleic acid tests. This enables hospitals to provide accurate and timely diagnoses, which are crucial for initiating appropriate treatment plans. In clinics, the focus is often on providing rapid and cost-effective diagnostic solutions. Clinics may rely on serological tests, such as ELISA, due to their ease of use and quick turnaround times. These tests allow clinicians to screen patients efficiently and refer them for further testing if necessary. The availability of point-of-care testing options is also increasing in clinics, enabling healthcare providers to diagnose Lyme disease on-site and reduce the need for multiple patient visits. Other settings, such as research laboratories and public health organizations, also play a role in the Global Lyme Disease Diagnostics Market. Research laboratories are involved in the development and validation of new diagnostic methods, contributing to the advancement of the field. Public health organizations focus on surveillance and monitoring of Lyme disease cases, utilizing diagnostic data to inform public health strategies and interventions. The integration of diagnostic tools across these various settings highlights the importance of a coordinated approach to Lyme disease management. By ensuring that accurate and reliable diagnostic options are available in hospitals, clinics, and other facilities, the Global Lyme Disease Diagnostics Market supports the early detection and treatment of Lyme disease, ultimately improving patient outcomes and reducing the burden of the disease on healthcare systems.
Global Lyme Disease Diagnostics Market Outlook:
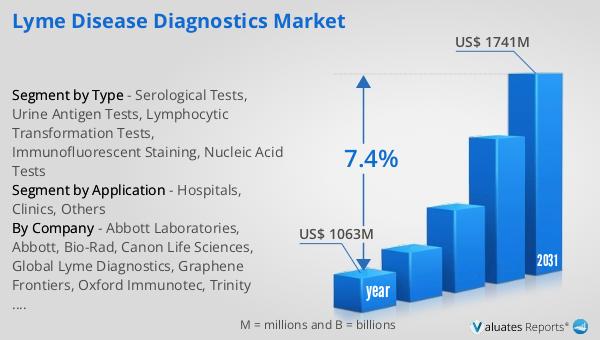
The global market for Lyme Disease Diagnostics was valued at $1,063 million in 2024 and is anticipated to grow to a revised size of $1,741 million by 2031, reflecting a compound annual growth rate (CAGR) of 7.4% during the forecast period. This growth is indicative of the increasing demand for effective diagnostic solutions as awareness and incidence of Lyme disease rise. In comparison, the global pharmaceutical market was valued at $1,475 billion in 2022, with a projected CAGR of 5% over the next six years. This highlights the relatively faster growth rate of the Lyme Disease Diagnostics Market, driven by technological advancements and increased research funding. Meanwhile, the chemical drug market is estimated to have grown from $1,005 billion in 2018 to $1,094 billion in 2022. The comparison underscores the dynamic nature of the Lyme Disease Diagnostics Market, which is expanding at a pace that outstrips some segments of the broader pharmaceutical industry. This growth trajectory reflects the critical need for improved diagnostic tools to combat Lyme disease and the significant opportunities for companies operating in this space.
| Report Metric | Details |
| Report Name | Lyme Disease Diagnostics Market |
| Accounted market size in year | US$ 1063 million |
| Forecasted market size in 2031 | US$ 1741 million |
| CAGR | 7.4% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Abbott Laboratories, Abbott, Bio-Rad, Canon Life Sciences, Global Lyme Diagnostics, Graphene Frontiers, Oxford Immunotec, Trinity Biotech |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |