What is Global Nitisinone Market?
The Global Nitisinone Market revolves around the pharmaceutical landscape where nitisinone, a medication primarily used to treat a rare genetic disorder called hereditary tyrosinemia type 1 (HT-1), plays a crucial role. This disorder affects the way the body breaks down the amino acid tyrosine, leading to serious liver and kidney problems if untreated. Nitisinone works by inhibiting an enzyme involved in the breakdown of tyrosine, thereby reducing the levels of toxic substances that can cause damage to the liver and kidneys. The market for nitisinone is driven by the increasing awareness and diagnosis of HT-1, advancements in genetic research, and the development of more effective treatment protocols. As a niche market, it is characterized by a limited number of players, with pharmaceutical companies focusing on innovation and strategic partnerships to enhance their product offerings and reach. The market's growth is also influenced by regulatory approvals and the expansion of healthcare infrastructure in emerging economies, which facilitate better access to treatment for patients. Overall, the Global Nitisinone Market is a specialized segment within the pharmaceutical industry, underscoring the importance of targeted therapies for rare diseases.
Orfadin, NITYR in the Global Nitisinone Market:
Orfadin and NITYR are two prominent brands within the Global Nitisinone Market, each offering unique formulations of nitisinone to treat hereditary tyrosinemia type 1 (HT-1). Orfadin, developed by Swedish Orphan Biovitrum AB (Sobi), was one of the first nitisinone products to gain approval and has been a cornerstone in the treatment of HT-1. It is available in both capsule and oral suspension forms, providing flexibility in administration, especially for pediatric patients who may have difficulty swallowing capsules. Orfadin's formulation is designed to ensure precise dosing and ease of use, which is critical in managing a chronic condition like HT-1. On the other hand, NITYR, developed by Cycle Pharmaceuticals, offers a tablet form of nitisinone that does not require refrigeration, unlike Orfadin's oral suspension. This feature makes NITYR particularly advantageous in regions with limited access to refrigeration, enhancing its accessibility and convenience for patients and caregivers. Both Orfadin and NITYR have undergone rigorous clinical trials to establish their efficacy and safety profiles, with studies demonstrating significant improvements in liver function and overall survival rates in patients with HT-1. The availability of these two brands provides healthcare professionals with options to tailor treatment plans based on individual patient needs and preferences. The competition between Orfadin and NITYR also drives innovation and improvements in patient care, as manufacturers strive to enhance their formulations and delivery methods. Furthermore, both brands are supported by patient assistance programs and educational initiatives aimed at raising awareness about HT-1 and the importance of early diagnosis and treatment. These efforts are crucial in ensuring that patients receive timely and effective care, ultimately improving their quality of life. As the Global Nitisinone Market continues to evolve, the presence of Orfadin and NITYR highlights the ongoing commitment to addressing the needs of patients with rare genetic disorders and the role of pharmaceutical innovation in transforming healthcare outcomes.
Adults, Children in the Global Nitisinone Market:
The usage of nitisinone in the Global Nitisinone Market spans across different age groups, including adults and children, each with specific considerations and treatment protocols. In adults, nitisinone is primarily used to manage hereditary tyrosinemia type 1 (HT-1), a condition that, if left untreated, can lead to severe liver and kidney complications. The treatment regimen for adults typically involves a daily dose of nitisinone, which helps to reduce the levels of toxic metabolites in the body, thereby preventing liver damage and improving overall health outcomes. Regular monitoring of liver function and tyrosine levels is essential to ensure the effectiveness of the treatment and to make necessary adjustments to the dosage. In children, the use of nitisinone is particularly critical, as early intervention can significantly alter the disease's progression and improve long-term prognosis. Pediatric patients often require a more tailored approach, with dosing adjusted based on weight and age to ensure optimal therapeutic outcomes. The availability of different formulations, such as oral suspensions and tablets, provides flexibility in administration, making it easier for caregivers to manage the treatment regimen. Additionally, the importance of dietary management cannot be overstated, as patients on nitisinone therapy need to adhere to a low-protein diet to minimize the risk of tyrosine accumulation and associated complications. This aspect of treatment requires collaboration between healthcare providers, dietitians, and families to ensure compliance and to address any nutritional concerns. The Global Nitisinone Market's focus on both adult and pediatric populations underscores the need for comprehensive care strategies that encompass medication, dietary management, and regular monitoring to achieve the best possible outcomes for patients with HT-1.
Global Nitisinone Market Outlook:
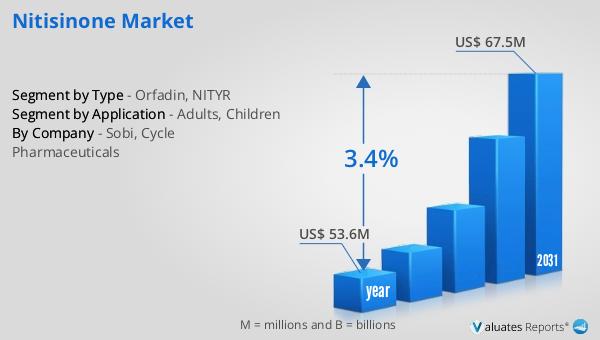
The worldwide market for nitisinone was estimated to be worth $53.6 million in 2024, and it is anticipated to expand to a revised valuation of $67.5 million by 2031, reflecting a compound annual growth rate (CAGR) of 3.4% over the forecast period. This growth trajectory highlights the increasing demand for nitisinone as a critical therapeutic option for hereditary tyrosinemia type 1 (HT-1), driven by factors such as improved diagnostic capabilities, greater awareness of the condition, and advancements in treatment protocols. The market's expansion is also supported by the development of new formulations and delivery methods that enhance patient convenience and adherence to treatment regimens. As healthcare systems around the world continue to evolve, there is a growing emphasis on addressing rare genetic disorders like HT-1, which in turn fuels the demand for effective therapies such as nitisinone. The projected growth of the Global Nitisinone Market reflects the ongoing commitment of pharmaceutical companies to innovate and improve patient outcomes, as well as the increasing recognition of the importance of targeted therapies in managing complex medical conditions. This market outlook underscores the potential for continued advancements in the field of rare disease treatment and the critical role that nitisinone plays in improving the lives of patients with HT-1.
| Report Metric | Details |
| Report Name | Nitisinone Market |
| Accounted market size in year | US$ 53.6 million |
| Forecasted market size in 2031 | US$ 67.5 million |
| CAGR | 3.4% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Sobi, Cycle Pharmaceuticals |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
