What is Global Rifaximin Market?
The Global Rifaximin Market is a segment of the pharmaceutical industry focused on the production and distribution of rifaximin, an antibiotic primarily used to treat gastrointestinal conditions. Rifaximin is a non-absorbable antibiotic, meaning it works within the gut without being absorbed into the bloodstream, making it effective for treating conditions like traveler’s diarrhea, irritable bowel syndrome with diarrhea (IBS-D), and hepatic encephalopathy. The market for rifaximin is driven by the increasing prevalence of these conditions, as well as the growing awareness of rifaximin's benefits among healthcare professionals and patients. Additionally, the market is influenced by the ongoing research and development activities aimed at expanding the therapeutic applications of rifaximin. The demand for rifaximin is also supported by its relatively favorable side effect profile compared to other antibiotics, which makes it a preferred choice for long-term treatment. As healthcare systems worldwide continue to focus on improving gastrointestinal health, the Global Rifaximin Market is expected to experience steady growth. The market is characterized by the presence of several key players who are engaged in strategic collaborations, mergers, and acquisitions to enhance their market presence and product offerings.
200 mg Tablets, 550 mg Tablets in the Global Rifaximin Market:
In the Global Rifaximin Market, two primary formulations are available: 200 mg tablets and 550 mg tablets. These formulations cater to different therapeutic needs and patient populations. The 200 mg tablets are typically prescribed for the treatment of traveler’s diarrhea caused by non-invasive strains of Escherichia coli. This dosage is effective in reducing the duration and severity of diarrhea, allowing patients to recover more quickly and resume their normal activities. The 200 mg tablets are often favored for their convenience and ease of administration, making them a popular choice for travelers who may be at risk of developing diarrhea during their trips. On the other hand, the 550 mg tablets are primarily used for the management of hepatic encephalopathy, a serious condition that affects the brain function of individuals with liver disease. The higher dosage is necessary to effectively reduce the production of ammonia and other toxins in the gut, which can contribute to the symptoms of hepatic encephalopathy. The 550 mg tablets are also used in the treatment of irritable bowel syndrome with diarrhea (IBS-D), where they help to alleviate symptoms such as abdominal pain, bloating, and frequent bowel movements. The choice between the 200 mg and 550 mg tablets depends on the specific condition being treated, the severity of symptoms, and the patient’s overall health status. Healthcare providers carefully assess these factors to determine the most appropriate dosage for each patient. The availability of these two formulations allows for greater flexibility in treatment, enabling healthcare providers to tailor therapy to the individual needs of their patients. In addition to their therapeutic benefits, both the 200 mg and 550 mg tablets are designed to be well-tolerated, with a low incidence of side effects. This makes them suitable for long-term use in chronic conditions such as hepatic encephalopathy and IBS-D. The tablets are also formulated to minimize the risk of antibiotic resistance, a significant concern in the treatment of bacterial infections. By targeting specific bacteria in the gut without affecting the broader microbiome, rifaximin helps to preserve the balance of beneficial bacteria, reducing the likelihood of resistance development. The Global Rifaximin Market is supported by ongoing research efforts aimed at optimizing the use of these formulations and exploring new therapeutic indications. Clinical trials and studies are conducted to evaluate the efficacy and safety of rifaximin in various patient populations, providing valuable data that informs clinical practice and guides treatment decisions. As a result, the market continues to evolve, with new insights and innovations contributing to the development of more effective and personalized treatment strategies. The availability of 200 mg and 550 mg tablets in the Global Rifaximin Market underscores the importance of providing diverse treatment options to meet the needs of patients with different gastrointestinal conditions. By offering these formulations, the market ensures that patients have access to effective and well-tolerated therapies that can improve their quality of life and support their overall health and well-being.
Hospital, Drug store in the Global Rifaximin Market:
The usage of rifaximin in hospitals and drug stores is a critical component of the Global Rifaximin Market, reflecting the widespread adoption of this antibiotic in clinical practice. In hospital settings, rifaximin is commonly used to manage patients with hepatic encephalopathy, a condition that requires careful monitoring and treatment to prevent serious complications. Hospitals rely on rifaximin as part of a comprehensive treatment plan that may also include dietary modifications, lactulose therapy, and other supportive measures. The use of rifaximin in hospitals is supported by clinical guidelines and evidence-based practices that highlight its efficacy in reducing the risk of recurrent episodes of hepatic encephalopathy. Hospital pharmacists and healthcare providers work closely to ensure that rifaximin is administered appropriately, taking into account factors such as the patient’s liver function, concurrent medications, and overall health status. In addition to hepatic encephalopathy, rifaximin is also used in hospitals to treat patients with irritable bowel syndrome with diarrhea (IBS-D) and traveler’s diarrhea. These conditions can significantly impact a patient’s quality of life, and timely treatment with rifaximin can help alleviate symptoms and improve outcomes. Hospitals may also use rifaximin as part of a broader strategy to manage antibiotic resistance, as its targeted action in the gut minimizes the risk of resistance development. In drug stores, rifaximin is available as a prescription medication, allowing patients to access this important treatment option in the community setting. Pharmacists play a key role in educating patients about the proper use of rifaximin, including the importance of adhering to the prescribed dosage and duration of therapy. They also provide guidance on potential side effects and interactions with other medications, ensuring that patients have the information they need to use rifaximin safely and effectively. Drug stores serve as a convenient access point for patients who require ongoing treatment with rifaximin, particularly those with chronic conditions such as hepatic encephalopathy and IBS-D. The availability of rifaximin in drug stores also supports continuity of care, as patients can easily obtain refills and maintain their treatment regimen as prescribed by their healthcare provider. The role of drug stores in the Global Rifaximin Market is further enhanced by the presence of knowledgeable pharmacists who can address patient concerns and provide personalized advice on medication management. Overall, the use of rifaximin in hospitals and drug stores highlights the importance of this antibiotic in the management of gastrointestinal conditions. Its availability in these settings ensures that patients have access to effective treatment options that can improve their health and quality of life. The collaboration between healthcare providers, pharmacists, and patients is essential to optimizing the use of rifaximin and achieving the best possible outcomes. As the Global Rifaximin Market continues to grow, the role of hospitals and drug stores in supporting patient access to rifaximin will remain a key focus, ensuring that this valuable treatment option is available to those who need it.
Global Rifaximin Market Outlook:
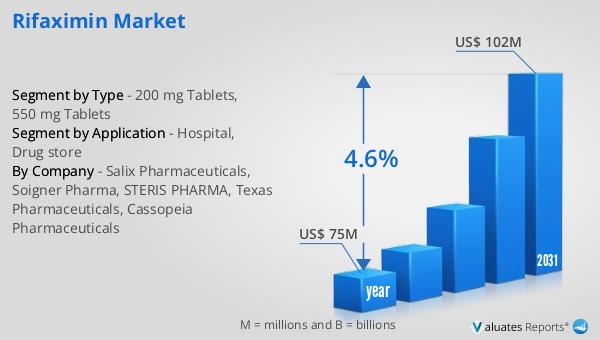
The global market for rifaximin was valued at $75 million in 2024, with projections indicating that it will reach a revised size of $102 million by 2031. This growth represents a compound annual growth rate (CAGR) of 4.6% over the forecast period. This upward trend in the market is driven by several factors, including the increasing prevalence of gastrointestinal conditions such as traveler’s diarrhea, irritable bowel syndrome with diarrhea (IBS-D), and hepatic encephalopathy. As awareness of rifaximin's benefits continues to grow among healthcare professionals and patients, the demand for this antibiotic is expected to rise. The market's expansion is also supported by ongoing research and development activities aimed at exploring new therapeutic applications for rifaximin and optimizing its use in existing indications. The relatively favorable side effect profile of rifaximin compared to other antibiotics makes it a preferred choice for long-term treatment, further contributing to market growth. Additionally, strategic collaborations, mergers, and acquisitions among key players in the market are enhancing their market presence and product offerings, driving further growth. As healthcare systems worldwide continue to focus on improving gastrointestinal health, the Global Rifaximin Market is poised for steady growth, providing valuable treatment options for patients with various gastrointestinal conditions.
| Report Metric | Details |
| Report Name | Rifaximin Market |
| Accounted market size in year | US$ 75 million |
| Forecasted market size in 2031 | US$ 102 million |
| CAGR | 4.6% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Salix Pharmaceuticals, Soigner Pharma, STERIS PHARMA, Texas Pharmaceuticals, Cassopeia Pharmaceuticals |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
