What is Global Adalimumab Market?
The Global Adalimumab Market refers to the worldwide industry focused on the production, distribution, and sale of Adalimumab, a biologic medication primarily used to treat various autoimmune diseases. Adalimumab is a monoclonal antibody that works by inhibiting tumor necrosis factor-alpha (TNF-alpha), a substance in the body that causes inflammation and is involved in the immune response. This market encompasses both the original branded version of Adalimumab and its biosimilar counterparts, which are essentially generic versions that are highly similar to the original product but are typically offered at a lower price. The market is driven by the increasing prevalence of autoimmune diseases such as rheumatoid arthritis, Crohn's disease, and psoriasis, which require effective long-term treatment options. Additionally, the expiration of patents for the original Adalimumab has opened the door for biosimilar products, further expanding the market. The competition between branded and biosimilar products, along with regulatory approvals and healthcare policies, plays a significant role in shaping the dynamics of the Global Adalimumab Market. As healthcare systems worldwide strive to manage costs while ensuring patient access to effective treatments, the market for Adalimumab and its biosimilars continues to evolve.
Adalimumab, Adalimumab Biosimilar in the Global Adalimumab Market:
Adalimumab is a biologic medication that has revolutionized the treatment of several chronic inflammatory conditions. It is a monoclonal antibody that specifically targets and neutralizes tumor necrosis factor-alpha (TNF-alpha), a cytokine involved in systemic inflammation. By inhibiting TNF-alpha, Adalimumab helps reduce inflammation and halt disease progression in conditions like rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, Crohn's disease, ulcerative colitis, and plaque psoriasis. The introduction of Adalimumab biosimilars has further expanded the market, offering more affordable options for patients and healthcare systems. Biosimilars are highly similar to the original biologic product in terms of safety, efficacy, and quality, but they are typically sold at a lower price, making them an attractive option for cost-conscious healthcare providers and patients. The development and approval of biosimilars are subject to rigorous regulatory standards to ensure they meet the same high standards as the original product. The availability of biosimilars has increased competition in the market, leading to potential cost savings and improved access to treatment for patients worldwide. The Global Adalimumab Market is characterized by a complex interplay of factors, including patent expirations, regulatory approvals, pricing strategies, and market competition. As patents for the original Adalimumab have expired in many regions, biosimilar manufacturers have entered the market, offering alternatives that are often more affordable. This has led to increased competition and pressure on pricing, benefiting healthcare systems and patients by potentially lowering treatment costs. However, the introduction of biosimilars also presents challenges, such as the need for healthcare providers to be educated about the similarities and differences between biosimilars and the original biologic, as well as the need for robust pharmacovigilance systems to monitor the safety and efficacy of these products in real-world settings. Despite these challenges, the adoption of Adalimumab biosimilars is expected to grow as healthcare systems seek to balance cost containment with the need to provide effective treatments for chronic inflammatory diseases. The Global Adalimumab Market is also influenced by regional variations in healthcare policies, reimbursement systems, and patient preferences. In some regions, biosimilars have been rapidly adopted due to supportive regulatory frameworks and incentives for healthcare providers to prescribe them. In other regions, the uptake of biosimilars has been slower, influenced by factors such as physician and patient familiarity with the original biologic, concerns about switching from the original product to a biosimilar, and differences in regulatory and reimbursement environments. Overall, the Global Adalimumab Market is a dynamic and evolving landscape, shaped by the interplay of scientific innovation, regulatory developments, market competition, and healthcare policy. As the market continues to grow, it holds the potential to improve access to effective treatments for patients with chronic inflammatory diseases, while also addressing the economic challenges faced by healthcare systems worldwide.
Adults, Children in the Global Adalimumab Market:
The Global Adalimumab Market plays a crucial role in providing effective treatment options for both adults and children suffering from various autoimmune diseases. In adults, Adalimumab is commonly prescribed for conditions such as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, Crohn's disease, ulcerative colitis, and plaque psoriasis. These conditions are characterized by chronic inflammation and can significantly impact a person's quality of life. Adalimumab works by targeting and neutralizing tumor necrosis factor-alpha (TNF-alpha), a key cytokine involved in the inflammatory process. By reducing inflammation, Adalimumab helps alleviate symptoms, improve physical function, and prevent disease progression in adults. The availability of Adalimumab biosimilars has further expanded treatment options for adults, offering more affordable alternatives that maintain the same safety and efficacy profile as the original biologic. This has led to increased access to treatment and potential cost savings for healthcare systems and patients. In children, Adalimumab is used to treat conditions such as juvenile idiopathic arthritis (JIA), pediatric Crohn's disease, and pediatric ulcerative colitis. These conditions can be particularly challenging to manage in children, as they can affect growth, development, and overall quality of life. Adalimumab provides an important treatment option for pediatric patients, helping to control inflammation, reduce symptoms, and improve long-term outcomes. The use of Adalimumab in children requires careful consideration of dosing and monitoring to ensure safety and efficacy. Pediatric patients may require different dosing regimens compared to adults, and healthcare providers must closely monitor for potential side effects and adjust treatment as needed. The introduction of Adalimumab biosimilars has also benefited pediatric patients by providing more cost-effective treatment options, which can be particularly important for families and healthcare systems managing the long-term care of children with chronic conditions. The Global Adalimumab Market is shaped by various factors, including regulatory approvals, pricing strategies, and market competition. In many regions, the availability of biosimilars has increased competition and led to potential cost savings, benefiting both adult and pediatric patients. However, the adoption of biosimilars also presents challenges, such as the need for healthcare providers to be educated about the similarities and differences between biosimilars and the original biologic, as well as the need for robust pharmacovigilance systems to monitor the safety and efficacy of these products in real-world settings. Despite these challenges, the Global Adalimumab Market continues to evolve, driven by the increasing prevalence of autoimmune diseases and the need for effective, affordable treatment options for both adults and children. As the market grows, it holds the potential to improve access to life-changing treatments for patients worldwide, while also addressing the economic challenges faced by healthcare systems.
Global Adalimumab Market Outlook:
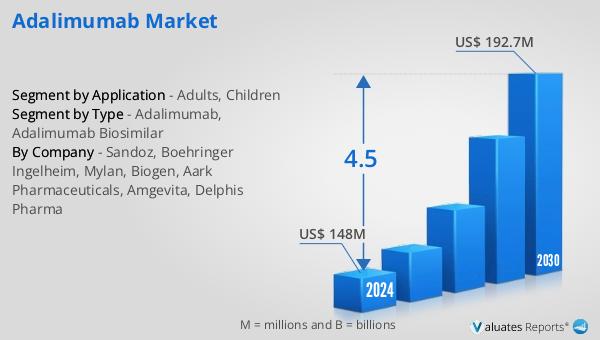
In 2024, the global market for Adalimumab was valued at approximately $154 million. This figure reflects the significant demand for Adalimumab, driven by its effectiveness in treating various autoimmune diseases. Over the years, the market has shown a steady growth trajectory, and it is anticipated to reach an estimated size of $209 million by 2031. This growth is projected to occur at a compound annual growth rate (CAGR) of 4.5% during the forecast period. The increase in market size can be attributed to several factors, including the rising prevalence of autoimmune diseases, the introduction of biosimilars, and the expansion of healthcare access in emerging markets. The availability of biosimilars has played a crucial role in driving market growth, as these products offer more affordable treatment options while maintaining the same safety and efficacy profile as the original biologic. This has led to increased competition in the market, resulting in potential cost savings for healthcare systems and patients. Additionally, advancements in healthcare infrastructure and increased awareness of autoimmune diseases have contributed to the growing demand for Adalimumab. As healthcare systems worldwide strive to manage costs while ensuring patient access to effective treatments, the Global Adalimumab Market is expected to continue its upward trajectory. The market's growth is also influenced by regional variations in healthcare policies, reimbursement systems, and patient preferences. In some regions, supportive regulatory frameworks and incentives for healthcare providers to prescribe biosimilars have accelerated market growth. In other regions, the uptake of biosimilars has been slower, influenced by factors such as physician and patient familiarity with the original biologic and differences in regulatory and reimbursement environments. Overall, the Global Adalimumab Market is a dynamic and evolving landscape, shaped by the interplay of scientific innovation, regulatory developments, market competition, and healthcare policy. As the market continues to grow, it holds the potential to improve access to effective treatments for patients with chronic inflammatory diseases, while also addressing the economic challenges faced by healthcare systems worldwide.
| Report Metric | Details |
| Report Name | Adalimumab Market |
| Accounted market size in year | US$ 154 million |
| Forecasted market size in 2031 | US$ 209 million |
| CAGR | 4.5% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | AbbVie, Amgen, Sandoz, Boehringer Ingelheim, Mylan, Biogen, Aark Pharmaceuticals, Amgevita, Delphis Pharma |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
