What is Phenylpropanolamine - Global Market?
Phenylpropanolamine is a compound that has been widely used in the pharmaceutical industry, primarily for its decongestant properties. It was once a common ingredient in over-the-counter cold and cough medications, helping to relieve nasal congestion by shrinking blood vessels in the nasal passages. However, due to concerns about its potential side effects, particularly the risk of hemorrhagic stroke, its use in human medications has been significantly restricted in many countries. Despite these restrictions, the global market for phenylpropanolamine remains active, driven by its applications in veterinary medicine and certain regulated human medications. The market is characterized by a diverse range of products, including tablets, capsules, and syrups, which are formulated to meet specific therapeutic needs. The demand for phenylpropanolamine is influenced by factors such as regulatory changes, advancements in pharmaceutical formulations, and the ongoing need for effective decongestant therapies. As a result, the market continues to evolve, with manufacturers focusing on innovation and compliance with safety standards to maintain their competitive edge. The global market for phenylpropanolamine is a dynamic landscape, reflecting the complex interplay of scientific, regulatory, and commercial forces.
Tablet, Capsule, Syrup in the Phenylpropanolamine - Global Market:
Phenylpropanolamine is available in various formulations, including tablets, capsules, and syrups, each designed to cater to different patient needs and preferences. Tablets are one of the most common forms, offering a convenient and precise dosage for users. They are typically used for their ease of administration and long shelf life. Tablets containing phenylpropanolamine are often combined with other active ingredients to enhance their therapeutic effects, such as antihistamines for allergy relief or analgesics for pain management. Capsules, on the other hand, provide an alternative for those who may have difficulty swallowing tablets. They are designed to dissolve quickly in the stomach, allowing for rapid absorption of the active ingredient. Capsules can also be formulated to release the drug over an extended period, providing sustained relief from symptoms. Syrups are particularly popular for pediatric and geriatric patients, as they offer a palatable and easily adjustable dosage form. The liquid nature of syrups allows for flexible dosing, which is especially important for children who require smaller or more precise doses. In the global market, these formulations are subject to stringent regulatory oversight to ensure their safety and efficacy. Manufacturers must adhere to good manufacturing practices and conduct rigorous testing to meet the standards set by health authorities. The choice of formulation often depends on factors such as patient demographics, the severity of symptoms, and the presence of any co-existing medical conditions. For instance, patients with chronic conditions may benefit from extended-release capsules, while those with acute symptoms might prefer the immediate relief provided by syrups. The global market for phenylpropanolamine-based products is also influenced by regional preferences and healthcare practices. In some regions, there may be a higher demand for certain formulations due to cultural or economic factors. For example, in areas where access to healthcare is limited, tablets may be preferred for their portability and ease of distribution. Conversely, in more developed markets, there may be a greater emphasis on innovative formulations that offer enhanced convenience or therapeutic benefits. As the market continues to grow, manufacturers are investing in research and development to create new and improved formulations that meet the evolving needs of patients and healthcare providers. This includes exploring novel delivery systems, such as transdermal patches or inhalers, which could offer additional benefits in terms of ease of use and patient compliance. Overall, the global market for phenylpropanolamine-based tablets, capsules, and syrups is a complex and dynamic environment, shaped by a multitude of factors that influence product development, regulatory compliance, and consumer preferences.
Human, Veterinary in the Phenylpropanolamine - Global Market:
Phenylpropanolamine is used in both human and veterinary medicine, although its applications and regulatory status differ significantly between the two fields. In human medicine, phenylpropanolamine was once widely used as a decongestant in over-the-counter cold and allergy medications. However, due to concerns about its potential to cause hemorrhagic stroke, its use has been restricted or banned in many countries. Despite these restrictions, phenylpropanolamine is still used in some prescription medications, particularly in formulations where its benefits outweigh the risks. These medications are typically used to treat conditions such as nasal congestion and sinusitis, where phenylpropanolamine's vasoconstrictive properties can provide significant relief. In veterinary medicine, phenylpropanolamine is primarily used to manage urinary incontinence in dogs. It works by increasing the tone of the urethral sphincter, helping to prevent involuntary leakage of urine. This application has proven to be highly effective, making phenylpropanolamine a valuable tool for veterinarians treating this common condition. The veterinary market for phenylpropanolamine is less restricted than the human market, allowing for a wider range of formulations and dosages. This flexibility enables veterinarians to tailor treatments to the specific needs of their patients, ensuring optimal outcomes. The global market for phenylpropanolamine in veterinary medicine is driven by factors such as the prevalence of urinary incontinence in dogs, advancements in veterinary pharmaceuticals, and the growing awareness of pet health and wellness. As pet ownership continues to rise worldwide, the demand for effective veterinary medications is expected to increase, further supporting the market for phenylpropanolamine. In both human and veterinary medicine, the use of phenylpropanolamine is subject to rigorous regulatory oversight to ensure its safety and efficacy. Manufacturers must comply with strict guidelines and conduct extensive testing to demonstrate the quality and reliability of their products. This includes monitoring for potential side effects and ensuring that formulations are appropriately labeled and dosed. Overall, the global market for phenylpropanolamine reflects the diverse and evolving needs of both human and veterinary healthcare, highlighting the importance of balancing therapeutic benefits with safety considerations.
Phenylpropanolamine - Global Market Outlook:
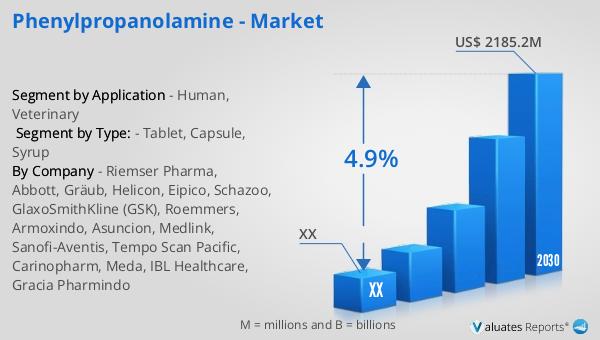
In the realm of veterinary medicine, phenylpropanolamine is primarily utilized to manage urinary incontinence in dogs. This application has proven to be highly effective, making it a valuable tool for veterinarians. The global market for phenylpropanolamine was valued at approximately $1,569 million in 2023. It is projected to grow to a revised size of $2,185.2 million by 2030, with a compound annual growth rate (CAGR) of 4.9% during the forecast period from 2024 to 2030. In comparison, the global pharmaceutical market was valued at $1,475 billion in 2022 and is expected to grow at a CAGR of 5% over the next six years. Meanwhile, the chemical drug market is anticipated to increase from $1,005 billion in 2018 to $1,094 billion in 2022. These figures highlight the significant role that phenylpropanolamine plays within the broader pharmaceutical landscape, particularly in the veterinary sector. The steady growth of the phenylpropanolamine market underscores its importance as a therapeutic agent, despite the challenges and regulatory hurdles it faces. As the market continues to evolve, manufacturers and healthcare providers must navigate these complexities to ensure the safe and effective use of phenylpropanolamine in both human and veterinary medicine.
| Report Metric | Details |
| Report Name | Phenylpropanolamine - Market |
| Forecasted market size in 2030 | US$ 2185.2 million |
| CAGR | 4.9% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Riemser Pharma, Abbott, Gräub, Helicon, Eipico, Schazoo, GlaxoSmithKline (GSK), Roemmers, Armoxindo, Asuncion, Medlink, Sanofi-Aventis, Tempo Scan Pacific, Carinopharm, Meda, IBL Healthcare, Gracia Pharmindo |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
