What is Renal Cell Carcinoma Therapy Devices - Global Market?
Renal cell carcinoma (RCC) therapy devices are specialized medical tools designed to treat renal cell carcinoma, a type of kidney cancer. These devices are part of a global market that focuses on providing innovative solutions for the effective management and treatment of RCC. The market encompasses a wide range of devices, including those used for surgical interventions, minimally invasive procedures, and advanced therapeutic techniques. The primary goal of these devices is to improve patient outcomes by offering precise and targeted treatment options that minimize damage to surrounding healthy tissues. As the prevalence of RCC continues to rise, the demand for effective therapy devices is also increasing, driving advancements in technology and expanding the market. The global market for RCC therapy devices is characterized by continuous research and development efforts, collaborations between medical device manufacturers and healthcare providers, and a growing emphasis on personalized medicine. These factors contribute to the development of more efficient and patient-friendly devices, ultimately enhancing the quality of care for individuals diagnosed with renal cell carcinoma. The market's growth is further supported by increasing awareness about early detection and treatment options, as well as the rising adoption of advanced medical technologies in healthcare settings worldwide.
Directed Energy-based Therapy Devices, Drug/Fluid Delivery and Infusion Therapy Devices in the Renal Cell Carcinoma Therapy Devices - Global Market:
Directed energy-based therapy devices, drug/fluid delivery, and infusion therapy devices play a crucial role in the global market for renal cell carcinoma (RCC) therapy devices. Directed energy-based therapy devices utilize focused energy sources, such as radiofrequency or microwave ablation, to precisely target and destroy cancerous cells in the kidney. These devices offer a minimally invasive alternative to traditional surgical procedures, reducing recovery time and minimizing complications for patients. By delivering energy directly to the tumor site, these devices can effectively shrink or eliminate tumors while preserving healthy kidney tissue. This approach is particularly beneficial for patients who are not suitable candidates for surgery due to underlying health conditions or the location of the tumor. Drug/fluid delivery and infusion therapy devices are essential components of RCC treatment, as they facilitate the administration of chemotherapy, immunotherapy, and targeted therapies. These devices ensure accurate dosing and controlled delivery of therapeutic agents, optimizing their effectiveness and minimizing side effects. Infusion pumps, catheters, and implantable ports are commonly used to deliver medications directly into the bloodstream, allowing for continuous or intermittent treatment as needed. The integration of advanced technologies, such as smart infusion systems and wireless monitoring, enhances the precision and safety of drug delivery, improving patient outcomes. The global market for RCC therapy devices is driven by the increasing prevalence of renal cell carcinoma, advancements in medical technology, and the growing demand for personalized treatment options. As healthcare providers strive to offer more effective and patient-centered care, the adoption of directed energy-based therapy devices and drug/fluid delivery systems is expected to rise. These devices not only improve the efficacy of RCC treatment but also enhance the overall patient experience by reducing the physical and emotional burden of cancer therapy. Furthermore, ongoing research and development efforts are focused on enhancing the capabilities of these devices, exploring new energy sources, and developing novel drug delivery mechanisms. The collaboration between medical device manufacturers, healthcare institutions, and research organizations is instrumental in driving innovation and expanding the range of available treatment options for RCC patients. As the global market for RCC therapy devices continues to evolve, it is poised to play a significant role in transforming the landscape of renal cell carcinoma treatment, offering hope and improved outcomes for patients worldwide.
Hospital, Clinic in the Renal Cell Carcinoma Therapy Devices - Global Market:
Renal cell carcinoma therapy devices are extensively used in hospitals and clinics, playing a vital role in the diagnosis, treatment, and management of renal cell carcinoma. In hospital settings, these devices are integral to providing comprehensive care for patients with RCC. Hospitals are equipped with advanced medical technologies and specialized personnel, making them ideal environments for the utilization of RCC therapy devices. Surgical interventions, such as nephrectomy or partial nephrectomy, are commonly performed in hospitals using state-of-the-art surgical devices and equipment. These procedures require precision and expertise, and the availability of advanced RCC therapy devices ensures optimal outcomes for patients. Additionally, hospitals often serve as centers for clinical trials and research, facilitating the development and evaluation of new RCC therapy devices and treatment protocols. In clinics, RCC therapy devices are used to provide outpatient care and follow-up treatment for patients with renal cell carcinoma. Clinics offer a more accessible and convenient setting for patients, allowing them to receive ongoing care and monitoring without the need for hospitalization. Directed energy-based therapy devices, such as radiofrequency ablation or cryoablation, are often utilized in clinics to perform minimally invasive procedures that can be completed on an outpatient basis. These devices enable clinicians to target and treat tumors with precision, reducing the need for extensive surgery and minimizing recovery time for patients. Drug/fluid delivery and infusion therapy devices are also commonly used in clinics to administer chemotherapy, immunotherapy, or targeted therapies. These devices ensure accurate dosing and controlled delivery of medications, allowing patients to receive treatment in a comfortable and familiar environment. The use of RCC therapy devices in hospitals and clinics is driven by the need to provide effective and patient-centered care for individuals with renal cell carcinoma. The integration of advanced technologies and innovative treatment approaches enhances the quality of care and improves patient outcomes. As the global market for RCC therapy devices continues to grow, hospitals and clinics are increasingly adopting these devices to meet the evolving needs of patients and healthcare providers. The collaboration between medical device manufacturers, healthcare institutions, and research organizations is crucial in advancing the development and utilization of RCC therapy devices, ultimately improving the standard of care for renal cell carcinoma patients worldwide.
Renal Cell Carcinoma Therapy Devices - Global Market Outlook:
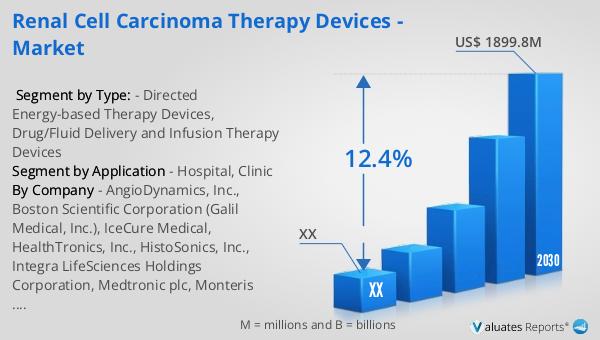
The global market for renal cell carcinoma therapy devices was valued at approximately $856 million in 2023. It is projected to grow significantly, reaching an estimated size of $1,899.8 million by 2030, with a compound annual growth rate (CAGR) of 12.4% during the forecast period from 2024 to 2030. This growth is indicative of the increasing demand for advanced medical devices and innovative treatment options for renal cell carcinoma. The broader medical devices market, valued at $603 billion in 2023, is also expected to experience growth, albeit at a more moderate CAGR of 5% over the next six years. The expansion of the RCC therapy devices market is driven by several factors, including the rising prevalence of renal cell carcinoma, advancements in medical technology, and the growing emphasis on personalized medicine. As healthcare providers and patients seek more effective and targeted treatment options, the demand for RCC therapy devices is expected to rise. The market's growth is further supported by increasing awareness about early detection and treatment options, as well as the rising adoption of advanced medical technologies in healthcare settings worldwide. The collaboration between medical device manufacturers, healthcare institutions, and research organizations is instrumental in driving innovation and expanding the range of available treatment options for RCC patients. As the global market for RCC therapy devices continues to evolve, it is poised to play a significant role in transforming the landscape of renal cell carcinoma treatment, offering hope and improved outcomes for patients worldwide.
| Report Metric | Details |
| Report Name | Renal Cell Carcinoma Therapy Devices - Market |
| Forecasted market size in 2030 | US$ 1899.8 million |
| CAGR | 12.4% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | AngioDynamics, Inc., Boston Scientific Corporation (Galil Medical, Inc.), IceCure Medical, HealthTronics, Inc., HistoSonics, Inc., Integra LifeSciences Holdings Corporation, Medtronic plc, Monteris Medical, Inc., Novocure GmbH, Varian Medical Systems, Inc. |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
