What is Global Sacubitril API Market?
The Global Sacubitril API Market is a segment of the pharmaceutical industry that focuses on the production and distribution of the active pharmaceutical ingredient (API) known as Sacubitril. Sacubitril is primarily used in combination with another drug, Valsartan, to treat heart failure. This combination is known as an angiotensin receptor-neprilysin inhibitor (ARNI) and is marketed under the brand name Entresto. The market for Sacubitril API is driven by the increasing prevalence of cardiovascular diseases, particularly heart failure, which is a leading cause of morbidity and mortality worldwide. As the global population ages and lifestyle-related health issues become more prevalent, the demand for effective heart failure treatments like Sacubitril is expected to rise. The market is characterized by a growing number of pharmaceutical companies investing in the development and production of Sacubitril API to meet this demand. Additionally, advancements in pharmaceutical manufacturing technologies and regulatory approvals are facilitating the expansion of this market. Overall, the Global Sacubitril API Market plays a crucial role in addressing the healthcare needs of patients with heart failure, contributing to improved patient outcomes and quality of life.
Above 98 %, Above 99 % in the Global Sacubitril API Market:
In the Global Sacubitril API Market, the purity levels of the API are critical factors that influence its application and effectiveness. Two common purity levels are "Above 98%" and "Above 99%." These purity levels refer to the percentage of Sacubitril present in the API, with the remainder consisting of impurities or other substances. The "Above 98%" purity level indicates that the API contains at least 98% Sacubitril, while the "Above 99%" purity level signifies a higher concentration of the active ingredient, with at least 99% purity. The choice between these purity levels depends on various factors, including the intended use of the API, regulatory requirements, and cost considerations. Higher purity levels are generally preferred for pharmaceutical applications, as they ensure greater efficacy and safety of the final drug product. However, achieving higher purity levels often involves more complex and costly manufacturing processes. In the context of the Global Sacubitril API Market, the demand for high-purity APIs is driven by the need for consistent and reliable drug formulations that meet stringent regulatory standards. Pharmaceutical companies must carefully balance the benefits of higher purity levels with the associated production costs to optimize their manufacturing processes and deliver effective treatments to patients. As the market continues to evolve, advancements in purification technologies and quality control measures are expected to enhance the availability and affordability of high-purity Sacubitril APIs, ultimately benefiting both manufacturers and patients.
Tablets, Others in the Global Sacubitril API Market:
The Global Sacubitril API Market finds its application in various pharmaceutical formulations, with tablets being one of the most common forms. Tablets are a popular dosage form due to their convenience, stability, and ease of administration. In the case of Sacubitril, tablets are often used in combination with Valsartan to create the ARNI drug Entresto, which is prescribed for the treatment of heart failure. The use of Sacubitril in tablet form allows for precise dosing and consistent delivery of the active ingredient, ensuring optimal therapeutic outcomes for patients. Additionally, tablets can be formulated with various excipients to enhance the stability, bioavailability, and patient acceptability of the drug. Beyond tablets, the Global Sacubitril API Market also encompasses other dosage forms, such as capsules, injectables, and oral solutions, to cater to diverse patient needs and preferences. These alternative formulations may be developed to address specific clinical requirements, such as rapid onset of action or improved patient compliance. For instance, oral solutions may be preferred for patients who have difficulty swallowing tablets, while injectables may be used in acute care settings where immediate drug action is necessary. The versatility of Sacubitril API in different dosage forms highlights its importance in the pharmaceutical industry and its potential to improve patient outcomes across various therapeutic areas. As the market continues to grow, ongoing research and development efforts are expected to expand the range of Sacubitril-based products available to healthcare providers and patients, further enhancing the treatment landscape for heart failure and related conditions.
Global Sacubitril API Market Outlook:
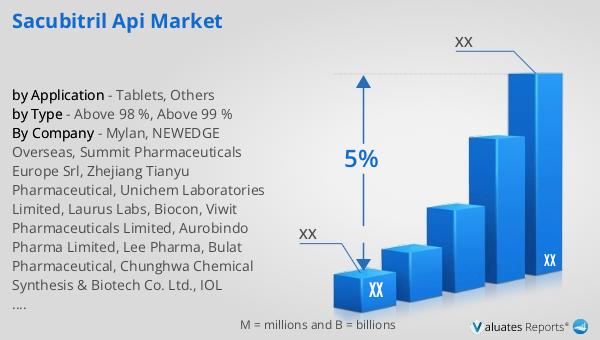
The outlook for the Global Sacubitril API Market can be contextualized within the broader pharmaceutical and chemical drug markets. In 2022, the global pharmaceutical market was valued at approximately 1,475 billion USD, with an anticipated compound annual growth rate (CAGR) of 5% over the next six years. This growth reflects the increasing demand for innovative and effective treatments across various therapeutic areas, including cardiovascular diseases. In comparison, the chemical drug market, which encompasses a wide range of active pharmaceutical ingredients, was estimated to grow from 1,005 billion USD in 2018 to 1,094 billion USD in 2022. This growth trajectory underscores the expanding role of chemical drugs, including Sacubitril, in addressing global healthcare needs. The Global Sacubitril API Market is poised to benefit from these broader industry trends, as the demand for heart failure treatments continues to rise. Pharmaceutical companies are likely to invest in the development and production of high-quality Sacubitril APIs to meet the growing needs of patients and healthcare providers. As the market evolves, factors such as regulatory approvals, technological advancements, and strategic partnerships will play a crucial role in shaping the future of the Sacubitril API Market. Ultimately, the market's growth will be driven by the ongoing pursuit of improved patient outcomes and the development of innovative therapies that address the complex challenges of heart failure and related conditions.
| Report Metric | Details |
| Report Name | Sacubitril API Market |
| CAGR | 5% |
| by Type |
|
| by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Mylan, NEWEDGE Overseas, Summit Pharmaceuticals Europe Srl, Zhejiang Tianyu Pharmaceutical, Unichem Laboratories Limited, Laurus Labs, Biocon, Viwit Pharmaceuticals Limited, Aurobindo Pharma Limited, Lee Pharma, Bulat Pharmaceutical, Chunghwa Chemical Synthesis & Biotech Co. Ltd., IOL Chemicals And Pharmaceuticals Limited |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |