What is Global Overactive Bladder (OAB) Bionic Drug Market?
The Global Overactive Bladder (OAB) Bionic Drug Market is a specialized segment within the pharmaceutical industry that focuses on developing and distributing medications designed to manage the symptoms of overactive bladder. This condition is characterized by a sudden, involuntary contraction of the muscle in the bladder wall, leading to an urgent need to urinate, frequent urination, and, in some cases, urinary incontinence. The market encompasses a range of therapeutic options, including anticholinergics, beta-3 adrenergic agonists like Mirabegron, and botulinum toxin (Botox) injections, among others. These treatments aim to alleviate the discomfort and inconvenience associated with OAB, improving the quality of life for those affected. The market is driven by an aging global population, increasing awareness of bladder health, and advancements in drug delivery systems. As the demand for effective OAB treatments grows, pharmaceutical companies are investing in research and development to create more efficient and patient-friendly solutions. The market's growth is also supported by healthcare providers' efforts to educate patients about OAB and the available treatment options, ensuring that more individuals seek medical advice and intervention for their symptoms.
Anticholinergics, Mirabegron, Botox, Others in the Global Overactive Bladder (OAB) Bionic Drug Market:
Anticholinergics are a class of drugs commonly used in the Global Overactive Bladder (OAB) Bionic Drug Market to manage symptoms by blocking the action of acetylcholine, a neurotransmitter that triggers bladder muscle contractions. By inhibiting these contractions, anticholinergics help reduce the frequency and urgency of urination, providing relief to patients. However, these drugs can have side effects such as dry mouth, constipation, and blurred vision, which may limit their use in some individuals. Despite these drawbacks, anticholinergics remain a cornerstone in OAB treatment due to their effectiveness in controlling symptoms. Mirabegron, on the other hand, represents a newer class of medication known as beta-3 adrenergic agonists. It works by relaxing the bladder muscle, increasing its storage capacity, and reducing the frequency of urination. Mirabegron is often preferred for patients who cannot tolerate the side effects of anticholinergics, as it generally has a more favorable side effect profile. Botox, or botulinum toxin, is another treatment option that involves injecting the toxin directly into the bladder muscle. This procedure temporarily paralyzes the muscle, reducing contractions and providing relief from OAB symptoms. Botox is typically considered for patients who have not responded to oral medications or who experience severe symptoms. While effective, Botox injections need to be repeated every few months, and there is a risk of urinary retention, which requires careful monitoring by healthcare providers. Other treatments in the OAB bionic drug market include neuromodulation therapies and combination therapies that utilize multiple drugs to achieve better symptom control. Neuromodulation involves stimulating nerves that control bladder function, either through implantable devices or external stimulation, to improve bladder control. Combination therapies may involve using anticholinergics with beta-3 adrenergic agonists or other medications to enhance efficacy and minimize side effects. The choice of treatment depends on various factors, including the severity of symptoms, patient preferences, and the presence of comorbid conditions. As research continues, the OAB bionic drug market is expected to evolve, with new drugs and treatment modalities emerging to address the diverse needs of patients. The ongoing development of personalized medicine approaches, which tailor treatments to individual patient profiles, holds promise for improving outcomes and patient satisfaction in managing overactive bladder.
Idiopathic Overactive Bladder, Neurogenic Overactive Bladder in the Global Overactive Bladder (OAB) Bionic Drug Market:
The Global Overactive Bladder (OAB) Bionic Drug Market plays a crucial role in managing two primary types of OAB: Idiopathic Overactive Bladder and Neurogenic Overactive Bladder. Idiopathic Overactive Bladder refers to cases where the cause of the bladder dysfunction is unknown. It is the most common form of OAB and can affect individuals of all ages, although it is more prevalent in older adults. The treatment for idiopathic OAB typically involves lifestyle modifications, behavioral therapies, and pharmacological interventions. Anticholinergics and Mirabegron are frequently prescribed to manage symptoms, with the choice of medication depending on the patient's response and tolerance to side effects. In some cases, Botox injections may be recommended for patients who do not respond to oral medications. Neurogenic Overactive Bladder, on the other hand, is caused by neurological conditions such as spinal cord injury, multiple sclerosis, or Parkinson's disease, which affect the nerves controlling bladder function. The management of neurogenic OAB is more complex and often requires a multidisciplinary approach. In addition to medications like anticholinergics and Mirabegron, patients may benefit from neuromodulation therapies, which involve stimulating the nerves that control bladder function to improve symptoms. For patients with severe neurogenic OAB, surgical interventions such as bladder augmentation or urinary diversion may be considered. The choice of treatment for neurogenic OAB depends on the underlying neurological condition, the severity of symptoms, and the patient's overall health status. In both idiopathic and neurogenic OAB, patient education and support are essential components of management. Healthcare providers play a critical role in helping patients understand their condition, the available treatment options, and the importance of adherence to prescribed therapies. As the Global OAB Bionic Drug Market continues to evolve, there is a growing emphasis on developing treatments that address the specific needs of patients with idiopathic and neurogenic OAB, improving their quality of life and reducing the burden of this condition.
Global Overactive Bladder (OAB) Bionic Drug Market Outlook:
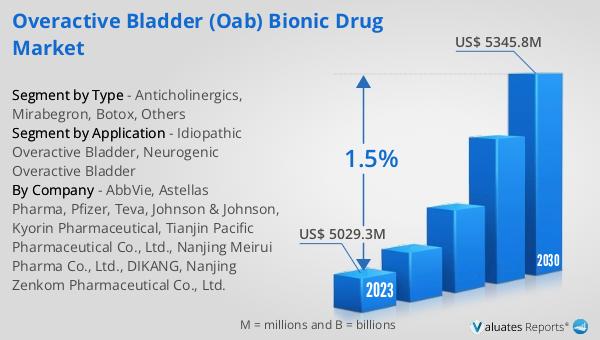
The worldwide market for Overactive Bladder (OAB) Bionic Drugs was valued at approximately $4,965 million in 2024. It is anticipated to expand to a revised size of $5,487 million by 2031, reflecting a compound annual growth rate (CAGR) of 1.5% throughout the forecast period. In contrast, the global pharmaceutical market was valued at $1,475 billion in 2022 and is projected to grow at a CAGR of 5% over the next six years. Meanwhile, the chemical drug market is expected to increase from $1,005 billion in 2018 to $1,094 billion by 2022. These figures highlight the relatively modest growth of the OAB Bionic Drug Market compared to the broader pharmaceutical and chemical drug markets. The slower growth rate of the OAB market may be attributed to various factors, including market saturation, the availability of generic alternatives, and the challenges associated with developing new and innovative treatments. Despite these challenges, the OAB Bionic Drug Market remains an important segment within the pharmaceutical industry, driven by the ongoing need for effective treatments for overactive bladder and the potential for advancements in drug delivery and personalized medicine.
| Report Metric | Details |
| Report Name | Overactive Bladder (OAB) Bionic Drug Market |
| Accounted market size in year | US$ 4965 million |
| Forecasted market size in 2031 | US$ 5487 million |
| CAGR | 1.5% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | AbbVie, Astellas Pharma, Pfizer, Teva, Johnson & Johnson, Kyorin Pharmaceutical, Tianjin Pacific Pharmaceutical Co., Ltd., Nanjing Meirui Pharma Co., Ltd., DIKANG, Nanjing Zenkom Pharmaceutical Co., Ltd. |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |