What is In Vitro Colorectal Cancer Testing - Global Market?
In vitro colorectal cancer testing is a crucial component of the global healthcare landscape, focusing on the early detection and diagnosis of colorectal cancer through laboratory-based tests. These tests are performed outside the human body, typically in a laboratory setting, using samples such as blood, stool, or tissue. The primary goal of these tests is to identify cancerous or precancerous conditions in the colon or rectum, which can significantly improve patient outcomes through early intervention. The global market for in vitro colorectal cancer testing is driven by the increasing prevalence of colorectal cancer, advancements in testing technologies, and growing awareness about the importance of early diagnosis. As healthcare systems worldwide strive to improve cancer detection rates, the demand for reliable and efficient in vitro testing methods continues to rise. This market encompasses a variety of test types, each offering unique benefits and challenges, and is characterized by ongoing research and development efforts aimed at enhancing test accuracy, reducing costs, and improving patient accessibility. The market's growth is further supported by government initiatives and healthcare policies that emphasize preventive care and early cancer detection, making in vitro colorectal cancer testing an essential tool in the fight against this prevalent disease.
Fecal Occult Blood Tests, Stool Biomarkers Tests, Blood Biomarker Tests in the In Vitro Colorectal Cancer Testing - Global Market:
Fecal occult blood tests (FOBT), stool biomarker tests, and blood biomarker tests are pivotal components of in vitro colorectal cancer testing, each offering distinct methodologies and benefits. FOBT is one of the most common and cost-effective screening methods used to detect hidden blood in the stool, which can be an early sign of colorectal cancer. This test is non-invasive and can be performed at home, making it accessible to a wide range of patients. However, its sensitivity can vary, and it may not detect all cases of colorectal cancer, necessitating follow-up procedures for confirmation. Stool biomarker tests, on the other hand, analyze specific genetic or protein markers in the stool that are associated with colorectal cancer. These tests offer higher sensitivity and specificity compared to traditional FOBT, providing a more accurate assessment of cancer risk. They are particularly useful for identifying early-stage cancers and precancerous lesions, which can significantly improve treatment outcomes. Blood biomarker tests represent another innovative approach, focusing on the detection of cancer-related biomarkers in the blood. These tests are minimally invasive and can be integrated into routine blood work, offering a convenient option for patients. Blood biomarker tests have the potential to detect colorectal cancer at an early stage, even before symptoms appear, making them a valuable tool for early diagnosis and intervention. Despite their promise, these tests are still under development and may require further validation to ensure their accuracy and reliability. The global market for in vitro colorectal cancer testing is witnessing a growing interest in these advanced testing methods, driven by the need for more precise and less invasive diagnostic tools. As research continues to advance, it is expected that these tests will become increasingly integrated into standard screening protocols, offering new hope for early detection and improved patient outcomes. The integration of these tests into healthcare systems worldwide is supported by technological advancements, increased funding for cancer research, and a growing emphasis on personalized medicine. As a result, the market for in vitro colorectal cancer testing is poised for significant growth, with these innovative testing methods playing a central role in shaping the future of cancer diagnostics.
Ambulatory Surgical Centers, Hospitals, Physical Examination Center in the In Vitro Colorectal Cancer Testing - Global Market:
In vitro colorectal cancer testing is utilized across various healthcare settings, including ambulatory surgical centers, hospitals, and physical examination centers, each offering unique advantages and challenges. Ambulatory surgical centers, known for their efficiency and convenience, often incorporate in vitro colorectal cancer testing as part of their routine screening services. These centers provide a streamlined approach to patient care, allowing for quick and accurate testing results that can facilitate timely interventions. The use of in vitro testing in these settings is particularly beneficial for patients who require regular monitoring or follow-up care, as it minimizes the need for invasive procedures and reduces overall healthcare costs. Hospitals, on the other hand, serve as comprehensive care facilities where in vitro colorectal cancer testing is integrated into a broader diagnostic and treatment framework. In these settings, patients have access to a wide range of medical expertise and resources, enabling a more thorough evaluation of test results and the development of personalized treatment plans. Hospitals often utilize advanced testing technologies and collaborate with research institutions to stay at the forefront of cancer diagnostics, ensuring that patients receive the most accurate and up-to-date care possible. Physical examination centers, which focus on preventive care and early detection, also play a crucial role in the utilization of in vitro colorectal cancer testing. These centers offer routine screenings as part of their health check-up packages, making it easier for individuals to access testing services and stay informed about their health status. By incorporating in vitro testing into their offerings, physical examination centers can help identify potential cancer risks early on, allowing for prompt intervention and improved patient outcomes. The integration of in vitro colorectal cancer testing across these diverse healthcare settings highlights the importance of early detection and the need for accessible, reliable diagnostic tools. As the global market for in vitro colorectal cancer testing continues to expand, these healthcare facilities will play a critical role in ensuring that patients receive timely and effective care, ultimately contributing to better health outcomes and a reduction in cancer-related mortality.
In Vitro Colorectal Cancer Testing - Global Market Outlook:
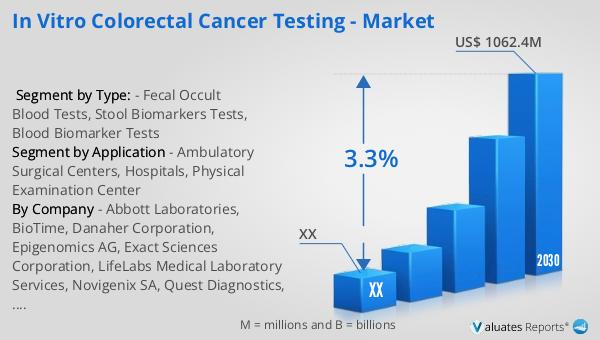
The global market for in vitro colorectal cancer testing was valued at approximately USD 851.2 million in 2023, with projections indicating a growth to USD 1,062.4 million by 2030. This represents a compound annual growth rate (CAGR) of 3.3% during the forecast period from 2024 to 2030. This growth is driven by several factors, including the increasing prevalence of colorectal cancer, advancements in testing technologies, and a growing emphasis on early detection and preventive care. The North American market, in particular, is expected to experience significant growth, although specific figures for this region were not provided. The expansion of the market is supported by government initiatives and healthcare policies that prioritize cancer screening and early diagnosis, as well as increased funding for research and development in this field. As healthcare systems worldwide continue to evolve, the demand for reliable and efficient in vitro colorectal cancer testing methods is expected to rise, contributing to the overall growth of the market. The integration of advanced testing technologies and personalized medicine approaches will further enhance the market's potential, offering new opportunities for innovation and improved patient outcomes. As a result, the global market for in vitro colorectal cancer testing is poised for continued growth and development, with a focus on improving accessibility, accuracy, and patient care.
| Report Metric | Details |
| Report Name | In Vitro Colorectal Cancer Testing - Market |
| Forecasted market size in 2030 | US$ 1062.4 million |
| CAGR | 3.3% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Abbott Laboratories, BioTime, Danaher Corporation, Epigenomics AG, Exact Sciences Corporation, LifeLabs Medical Laboratory Services, Novigenix SA, Quest Diagnostics, Sysmex Corporation |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
