What is Global Ophthalmic Medical Devices Market?
The Global Ophthalmic Medical Devices Market is a dynamic and rapidly evolving sector within the broader healthcare industry. This market encompasses a wide range of devices designed to diagnose, monitor, and treat various eye-related conditions and diseases. These devices are crucial for maintaining eye health and improving the quality of life for individuals with visual impairments. The market includes products such as contact lenses, intraocular lenses, ophthalmic surgical devices, diagnostic equipment, and vision care products. With the increasing prevalence of eye disorders like cataracts, glaucoma, and age-related macular degeneration, the demand for advanced ophthalmic devices is on the rise. Technological advancements, such as the development of minimally invasive surgical techniques and innovative diagnostic tools, are driving growth in this market. Additionally, the aging global population and the rising awareness of eye health are contributing to the expansion of the ophthalmic medical devices market. As healthcare systems worldwide continue to prioritize eye care, the market is expected to witness sustained growth, offering numerous opportunities for manufacturers and healthcare providers to enhance patient outcomes and address the growing needs of the global population.
Class I Medical Device, Class II Medical Devices, Class III Medical Devices in the Global Ophthalmic Medical Devices Market:
In the realm of the Global Ophthalmic Medical Devices Market, medical devices are categorized into three classes based on their risk level and regulatory requirements: Class I, Class II, and Class III. Class I medical devices are considered low-risk and are subject to the least regulatory control. These devices typically include simple ophthalmic tools and accessories that do not pose significant risks to patients. Examples of Class I devices in the ophthalmic sector might include basic diagnostic instruments like eye charts and non-invasive tools used for routine eye examinations. These devices are generally exempt from pre-market notification requirements, allowing for easier market entry and distribution.
Hospital, Eye Clinic, Other in the Global Ophthalmic Medical Devices Market:
Class II medical devices are of moderate risk and require more regulatory oversight than Class I devices. They often include products that are more complex and may come into contact with the eye or surrounding tissues. In the ophthalmic field, Class II devices might encompass diagnostic equipment such as tonometers, which measure intraocular pressure, and slit lamps, which provide detailed views of the eye's structures. These devices must comply with specific performance standards and may require pre-market notification to ensure their safety and effectiveness. The regulatory process for Class II devices is designed to provide a reasonable assurance of safety and effectiveness while allowing for innovation and technological advancements.
Global Ophthalmic Medical Devices Market Outlook:
Class III medical devices are considered high-risk and are subject to the most stringent regulatory controls. These devices are often life-sustaining or life-supporting and may pose significant risks if they fail. In the ophthalmic sector, Class III devices typically include advanced surgical instruments and implantable devices such as intraocular lenses used in cataract surgery. These devices require extensive clinical testing and pre-market approval to demonstrate their safety and efficacy. The regulatory process for Class III devices is rigorous, involving detailed evaluations of clinical data and manufacturing processes to ensure that they meet the highest standards of quality and safety. The development and approval of Class III devices are critical for addressing complex eye conditions and improving patient outcomes.
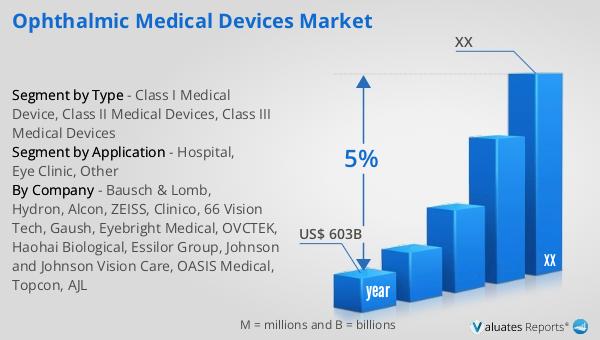
| Report Metric | Details |
| Report Name | Ophthalmic Medical Devices Market |
| Accounted market size in year | US$ 603 billion |
| CAGR | 5% |
| Base Year | year |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Bausch & Lomb, Hydron, Alcon, ZEISS, Clinico, 66 Vision Tech, Gaush, Eyebright Medical, OVCTEK, Haohai Biological, Essilor Group, Johnson and Johnson Vision Care, OASIS Medical, Topcon, AJL |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
