What is Global Cerebral Thrombectomy Device Market?
The Global Cerebral Thrombectomy Device Market is a specialized segment within the broader medical device industry, focusing on devices designed to remove blood clots from the brain's blood vessels. These devices are crucial in treating acute ischemic strokes, which occur when a blood clot obstructs blood flow to the brain, leading to potential brain damage or death if not promptly addressed. The market for these devices has been expanding due to the increasing prevalence of stroke cases worldwide, driven by factors such as aging populations, sedentary lifestyles, and rising incidences of conditions like hypertension and diabetes. Technological advancements have also played a significant role in the market's growth, with innovations leading to more effective and safer thrombectomy devices. As healthcare systems globally strive to improve stroke management and outcomes, the demand for cerebral thrombectomy devices is expected to continue rising. These devices not only offer a lifeline to patients experiencing strokes but also represent a critical component of modern medical interventions aimed at reducing the long-term impacts of such medical emergencies. The market's growth is further supported by increased awareness and training among healthcare professionals, ensuring that these life-saving devices are used effectively in clinical settings.
Stent Retrievers, Aspiration Catheters, Balloon Guide Catheters, Others in the Global Cerebral Thrombectomy Device Market:
Stent retrievers, aspiration catheters, balloon guide catheters, and other devices form the backbone of the Global Cerebral Thrombectomy Device Market, each playing a unique role in the treatment of ischemic strokes. Stent retrievers are among the most commonly used devices in thrombectomy procedures. They are designed to be inserted into the blocked artery, where they expand to capture and remove the clot. The design of stent retrievers allows them to effectively restore blood flow by mechanically engaging and extracting the clot, making them a preferred choice in many clinical scenarios. Aspiration catheters, on the other hand, work by directly suctioning the clot out of the artery. These devices are often used in conjunction with stent retrievers to enhance the efficacy of the thrombectomy procedure. Aspiration catheters are valued for their ability to remove clots quickly and efficiently, reducing the time the brain is deprived of blood flow. Balloon guide catheters are another critical component, often used to temporarily block blood flow in the artery during the procedure. This temporary occlusion helps to prevent the clot from breaking apart and traveling further into the brain, which could cause additional complications. The use of balloon guide catheters can improve the success rate of thrombectomy procedures by stabilizing the clot and facilitating its removal. Other devices in the market include various types of microcatheters and guidewires, which assist in navigating the complex vascular pathways of the brain. These tools are essential for ensuring that the primary thrombectomy devices can reach the site of the clot safely and effectively. The development of these devices has been driven by a deep understanding of the brain's vascular anatomy and the need for precision in stroke interventions. Each of these devices has undergone rigorous testing and refinement to ensure they meet the high standards required for medical use. The integration of these devices into comprehensive stroke treatment protocols has significantly improved patient outcomes, reducing the risk of long-term disability and enhancing recovery prospects. As the market continues to evolve, ongoing research and development efforts are focused on further improving the design and functionality of these devices, with the aim of making thrombectomy procedures even more effective and accessible. The collaboration between medical device manufacturers, healthcare providers, and researchers is crucial in driving innovation and ensuring that the latest advancements are translated into clinical practice. This synergy is vital for addressing the growing global burden of stroke and improving the quality of life for patients affected by this debilitating condition.
Hospital, Clinic, Others in the Global Cerebral Thrombectomy Device Market:
The usage of cerebral thrombectomy devices is primarily concentrated in hospitals, clinics, and other healthcare settings, each playing a pivotal role in the management of acute ischemic strokes. Hospitals are the primary setting for thrombectomy procedures, given their access to advanced imaging technologies and multidisciplinary teams of specialists. In a hospital environment, patients experiencing a stroke can be rapidly assessed using imaging techniques such as CT or MRI scans to determine the location and severity of the clot. Once identified, a team of neurologists, radiologists, and interventional specialists can collaborate to perform the thrombectomy procedure using the appropriate devices. The hospital setting provides the necessary infrastructure and expertise to handle potential complications and ensure optimal patient care throughout the procedure and recovery process. Clinics, particularly those specializing in neurology or stroke care, also play a significant role in the utilization of thrombectomy devices. While clinics may not have the same level of resources as hospitals, they often serve as critical points of initial assessment and referral for stroke patients. In some cases, clinics equipped with the necessary facilities and expertise may perform thrombectomy procedures, particularly in regions where access to larger hospitals is limited. The role of clinics in stroke management underscores the importance of having trained personnel and appropriate equipment available in diverse healthcare settings. Other healthcare facilities, including specialized stroke centers and rehabilitation units, contribute to the broader ecosystem of stroke care. These centers often focus on post-procedural care and rehabilitation, helping patients recover and regain function after a stroke. The integration of thrombectomy devices into these settings highlights the importance of a comprehensive approach to stroke management, where acute intervention is complemented by ongoing care and support. The widespread adoption of thrombectomy devices across various healthcare settings is driven by the recognition of their effectiveness in improving stroke outcomes. By restoring blood flow to the brain quickly and efficiently, these devices can significantly reduce the risk of long-term disability and enhance recovery prospects for stroke patients. The collaboration between different healthcare providers and the seamless integration of thrombectomy devices into stroke care protocols are essential for maximizing the benefits of these life-saving interventions. As awareness of the importance of timely and effective stroke treatment continues to grow, the demand for cerebral thrombectomy devices is expected to increase across all healthcare settings. This trend underscores the need for ongoing investment in training, infrastructure, and research to ensure that these devices are used to their full potential in improving patient outcomes.
Global Cerebral Thrombectomy Device Market Outlook:
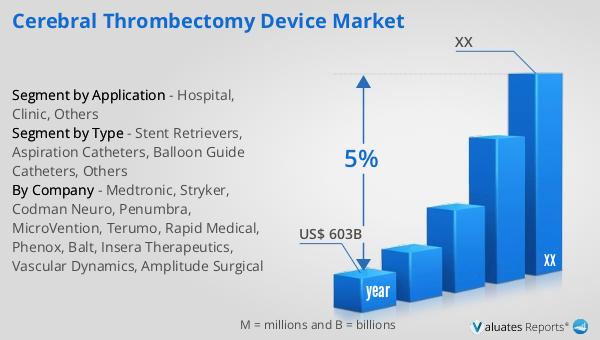
Based on our analysis, the global market for medical devices is projected to reach approximately $603 billion in 2023. This substantial market size reflects the growing demand for innovative medical technologies and devices across various healthcare sectors. Over the next six years, the market is expected to experience a steady growth rate, with a compound annual growth rate (CAGR) of 5%. This growth trajectory is indicative of the increasing reliance on medical devices to enhance patient care, improve diagnostic accuracy, and facilitate advanced treatment options. The expansion of the medical device market is driven by several factors, including technological advancements, an aging global population, and the rising prevalence of chronic diseases. As healthcare systems worldwide strive to improve patient outcomes and reduce healthcare costs, the adoption of cutting-edge medical devices is becoming increasingly important. The market's growth is also supported by regulatory frameworks that encourage innovation and ensure the safety and efficacy of medical devices. As the industry continues to evolve, stakeholders across the healthcare ecosystem, including manufacturers, healthcare providers, and policymakers, are working collaboratively to address challenges and seize opportunities in this dynamic market. The ongoing development and commercialization of new medical devices are expected to play a crucial role in shaping the future of healthcare delivery and improving the quality of life for patients worldwide.
| Report Metric | Details |
| Report Name | Cerebral Thrombectomy Device Market |
| Accounted market size in year | US$ 603 billion |
| CAGR | 5% |
| Base Year | year |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Medtronic, Stryker, Codman Neuro, Penumbra, MicroVention, Terumo, Rapid Medical, Phenox, Balt, Insera Therapeutics, Vascular Dynamics, Amplitude Surgical |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
