What is Global Veterinary Pharmacovigilance Market?
The Global Veterinary Pharmacovigilance Market is a specialized sector within the broader field of veterinary medicine that focuses on the monitoring, evaluation, and prevention of adverse effects associated with veterinary drugs. This market plays a crucial role in ensuring the safety and efficacy of medications used in animals, including both companion animals like dogs and cats, and farm animals such as cattle, pigs, and poultry. Veterinary pharmacovigilance involves the collection and analysis of data related to adverse drug reactions (ADRs) and other drug-related problems. This information is then used to improve drug safety, inform regulatory decisions, and guide the development of new veterinary pharmaceuticals. The market encompasses various stakeholders, including pharmaceutical companies, regulatory authorities, veterinarians, and pet owners, all working together to ensure that veterinary drugs are safe and effective for animal health. The increasing awareness about animal health and welfare, coupled with stringent regulatory requirements, is driving the growth of this market globally.
In-house, Contract Outsourcing in the Global Veterinary Pharmacovigilance Market:
In the Global Veterinary Pharmacovigilance Market, there are two primary operational models: in-house and contract outsourcing. In-house pharmacovigilance refers to the practice where pharmaceutical companies manage all aspects of drug safety monitoring internally. This includes the collection of adverse event reports, data analysis, and regulatory compliance. Companies that opt for in-house pharmacovigilance typically have dedicated teams of experts who are responsible for ensuring that all safety data is accurately recorded and reported. This model allows for greater control over the pharmacovigilance process and can lead to more efficient decision-making. However, it also requires significant investment in terms of resources and infrastructure. On the other hand, contract outsourcing involves partnering with external service providers to handle pharmacovigilance activities. These third-party organizations specialize in drug safety monitoring and offer a range of services, including data collection, analysis, and regulatory reporting. Outsourcing can be a cost-effective solution for companies that lack the resources or expertise to manage pharmacovigilance in-house. It allows companies to leverage the expertise of specialized service providers and can lead to more comprehensive and accurate safety monitoring. However, it also requires careful selection and management of outsourcing partners to ensure that all regulatory requirements are met. Both in-house and contract outsourcing models have their advantages and disadvantages, and the choice between them often depends on the specific needs and capabilities of the pharmaceutical company. In recent years, there has been a growing trend towards hybrid models, where companies combine in-house expertise with outsourced services to achieve a balance between control and cost-efficiency. This approach allows companies to maintain oversight of critical pharmacovigilance activities while benefiting from the specialized skills and resources of external providers. Overall, the choice between in-house and contract outsourcing in the Global Veterinary Pharmacovigilance Market is influenced by factors such as the size of the company, the complexity of its product portfolio, and its strategic priorities.
Companion Animals, Farm Animals in the Global Veterinary Pharmacovigilance Market:
The Global Veterinary Pharmacovigilance Market is essential for ensuring the safety and efficacy of medications used in both companion animals and farm animals. For companion animals, such as dogs, cats, and other pets, pharmacovigilance plays a critical role in monitoring adverse drug reactions and ensuring that medications are safe for use. Pet owners are increasingly aware of the importance of their pets' health and are more likely to report any adverse effects of medications to their veterinarians. This data is then collected and analyzed to identify potential safety issues and improve drug formulations. The growing trend of pet humanization, where pets are considered family members, has led to increased demand for safe and effective veterinary drugs, further driving the need for robust pharmacovigilance systems. In the case of farm animals, such as cattle, pigs, and poultry, veterinary pharmacovigilance is crucial for maintaining the health and productivity of livestock. Adverse drug reactions in farm animals can have significant economic implications, affecting not only the health of the animals but also the profitability of farming operations. Effective pharmacovigilance ensures that any potential safety issues are identified and addressed promptly, minimizing the risk of widespread health problems in livestock populations. Additionally, regulatory authorities require comprehensive pharmacovigilance data to approve and monitor veterinary drugs used in food-producing animals, ensuring that these medications do not pose a risk to human health through the food chain. The use of pharmacovigilance in both companion and farm animals highlights the importance of a coordinated approach to drug safety monitoring. By collecting and analyzing data from a wide range of sources, including veterinarians, pet owners, and farmers, the Global Veterinary Pharmacovigilance Market can identify trends and patterns that may indicate potential safety issues. This information is then used to inform regulatory decisions, guide the development of new drugs, and improve the overall safety and efficacy of veterinary medications.
Global Veterinary Pharmacovigilance Market Outlook:
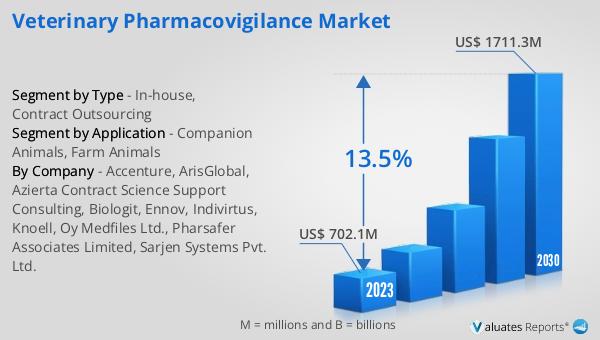
The global Veterinary Pharmacovigilance market was valued at US$ 702.1 million in 2023 and is anticipated to reach US$ 1711.3 million by 2030, witnessing a CAGR of 13.5% during the forecast period 2024-2030. This significant growth reflects the increasing importance of pharmacovigilance in ensuring the safety and efficacy of veterinary drugs. As awareness about animal health and welfare continues to rise, there is a growing demand for robust pharmacovigilance systems that can effectively monitor and manage adverse drug reactions. The market's expansion is also driven by stringent regulatory requirements that mandate comprehensive safety monitoring for veterinary medications. Pharmaceutical companies, regulatory authorities, veterinarians, and pet owners all play a crucial role in this process, working together to ensure that veterinary drugs are safe and effective for animal health. The projected growth of the market underscores the critical role of pharmacovigilance in the veterinary sector and highlights the need for continued investment in this area to support the development of safe and effective veterinary medications.
| Report Metric | Details |
| Report Name | Veterinary Pharmacovigilance Market |
| Accounted market size in 2023 | US$ 702.1 million |
| Forecasted market size in 2030 | US$ 1711.3 million |
| CAGR | 13.5% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Accenture, ArisGlobal, Azierta Contract Science Support Consulting, Biologit, Ennov, Indivirtus, Knoell, Oy Medfiles Ltd., Pharsafer Associates Limited, Sarjen Systems Pvt. Ltd. |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
