What is Global Veterinary CRO and CDMO Market?
The Global Veterinary CRO and CDMO Market is a specialized segment within the broader veterinary healthcare industry. It encompasses organizations that provide contract research and manufacturing services specifically for veterinary products. CROs, or Contract Research Organizations, focus on conducting research and clinical trials to develop new veterinary medicines and treatments. CDMOs, or Contract Development and Manufacturing Organizations, handle the production and development of these veterinary products. This market is crucial for advancing veterinary science, ensuring that animals receive the best possible care through innovative treatments and medications. The demand for these services is driven by the increasing pet ownership, rising awareness about animal health, and the need for advanced veterinary treatments. As a result, the Global Veterinary CRO and CDMO Market is experiencing significant growth, with companies investing heavily in research and development to meet the evolving needs of the veterinary industry.
Contract Development &Manufacturing Organizations (CDMO), Contract Research Organizations (CRO) in the Global Veterinary CRO and CDMO Market:
Contract Development and Manufacturing Organizations (CDMOs) and Contract Research Organizations (CROs) play pivotal roles in the Global Veterinary CRO and CDMO Market. CDMOs are specialized firms that provide comprehensive services from drug development to manufacturing. They assist veterinary pharmaceutical companies by offering expertise in formulation development, process optimization, and large-scale production. This allows veterinary companies to focus on their core competencies while leveraging the technical capabilities of CDMOs to bring new products to market efficiently. On the other hand, CROs are dedicated to conducting research and clinical trials. They design and execute studies to evaluate the safety and efficacy of new veterinary drugs and treatments. CROs manage various phases of clinical trials, from preclinical research to post-market surveillance, ensuring that new veterinary products meet regulatory standards and are safe for animal use. The collaboration between veterinary pharmaceutical companies and CROs/CDMOs is essential for the successful development and commercialization of veterinary products. These organizations provide the necessary infrastructure, expertise, and resources to navigate the complex regulatory landscape and bring innovative treatments to market. The Global Veterinary CRO and CDMO Market is characterized by a high level of specialization and technical expertise. CDMOs offer tailored solutions for veterinary drug development, including formulation development, analytical testing, and manufacturing scale-up. They employ state-of-the-art technologies and adhere to stringent quality standards to ensure the production of high-quality veterinary products. CROs, on the other hand, focus on designing and conducting clinical trials that generate robust data on the safety and efficacy of new veterinary treatments. They work closely with veterinary pharmaceutical companies to develop study protocols, recruit animal subjects, and collect and analyze data. The insights gained from these trials are critical for obtaining regulatory approvals and bringing new veterinary products to market. The Global Veterinary CRO and CDMO Market is driven by several factors, including the increasing demand for advanced veterinary treatments, the growing pet population, and the rising awareness about animal health. Veterinary pharmaceutical companies are investing heavily in research and development to meet the evolving needs of the veterinary industry. This has led to a surge in demand for the specialized services offered by CROs and CDMOs. These organizations play a crucial role in accelerating the development and commercialization of new veterinary products, ultimately improving the quality of care for animals. The collaboration between veterinary pharmaceutical companies and CROs/CDMOs is essential for advancing veterinary science and ensuring that animals receive the best possible treatments.
Medicines, Medical Devices in the Global Veterinary CRO and CDMO Market:
The Global Veterinary CRO and CDMO Market has significant applications in the development of veterinary medicines and medical devices. In the realm of veterinary medicines, CROs and CDMOs are instrumental in the entire lifecycle of drug development. CROs conduct preclinical research to identify promising drug candidates and assess their safety and efficacy in animal models. They design and execute clinical trials to evaluate the therapeutic potential of these candidates in target animal species. This involves recruiting animal subjects, administering the investigational drugs, and collecting data on their effects. The data generated from these trials are critical for obtaining regulatory approvals and ensuring that the new veterinary medicines are safe and effective for animal use. CDMOs, on the other hand, focus on the development and manufacturing aspects of veterinary medicines. They provide expertise in formulation development, ensuring that the drug is stable, bioavailable, and palatable for animals. CDMOs also handle the scale-up of manufacturing processes, producing the drug in large quantities while maintaining high quality standards. This is particularly important for veterinary medicines, as they often require specialized formulations and delivery methods to ensure proper administration and efficacy in animals. In the area of medical devices, the Global Veterinary CRO and CDMO Market also plays a crucial role. CROs conduct research and clinical trials to evaluate the safety and efficacy of new veterinary medical devices. This includes devices such as diagnostic tools, surgical instruments, and therapeutic devices. CROs design studies to assess the performance of these devices in real-world veterinary settings, collecting data on their effectiveness and safety. This data is essential for obtaining regulatory approvals and ensuring that the devices meet the necessary standards for veterinary use. CDMOs, in turn, provide development and manufacturing services for veterinary medical devices. They assist in the design and prototyping of new devices, ensuring that they meet the specific needs of veterinary practitioners. CDMOs also handle the production of these devices, employing advanced manufacturing techniques to produce high-quality products. This includes ensuring that the devices are durable, reliable, and safe for use in animals. The collaboration between veterinary pharmaceutical companies and CROs/CDMOs is essential for the successful development and commercialization of veterinary medicines and medical devices. These organizations provide the necessary infrastructure, expertise, and resources to navigate the complex regulatory landscape and bring innovative products to market. The Global Veterinary CRO and CDMO Market is driven by the increasing demand for advanced veterinary treatments and medical devices, the growing pet population, and the rising awareness about animal health. Veterinary pharmaceutical companies are investing heavily in research and development to meet the evolving needs of the veterinary industry. This has led to a surge in demand for the specialized services offered by CROs and CDMOs. These organizations play a crucial role in accelerating the development and commercialization of new veterinary products, ultimately improving the quality of care for animals.
Global Veterinary CRO and CDMO Market Outlook:
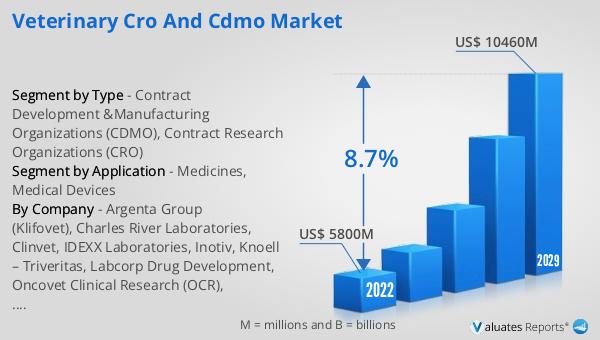
The global Veterinary CRO and CDMO market was valued at US$ 5800 million in 2023 and is anticipated to reach US$ 10460 million by 2030, witnessing a CAGR of 8.7% during the forecast period 2024-2030. This significant growth reflects the increasing demand for advanced veterinary treatments and the rising awareness about animal health. The market's expansion is driven by several factors, including the growing pet population, the need for innovative veterinary medicines and medical devices, and the investments in research and development by veterinary pharmaceutical companies. The collaboration between veterinary pharmaceutical companies and CROs/CDMOs is essential for advancing veterinary science and ensuring that animals receive the best possible care. These organizations provide the necessary infrastructure, expertise, and resources to navigate the complex regulatory landscape and bring innovative treatments to market. The Global Veterinary CRO and CDMO Market is characterized by a high level of specialization and technical expertise, with CDMOs offering tailored solutions for veterinary drug development and CROs focusing on designing and conducting clinical trials. The insights gained from these trials are critical for obtaining regulatory approvals and bringing new veterinary products to market. The market's growth is a testament to the importance of these specialized services in improving the quality of care for animals and advancing veterinary science.
| Report Metric | Details |
| Report Name | Veterinary CRO and CDMO Market |
| Accounted market size in 2023 | US$ 5800 million |
| Forecasted market size in 2030 | US$ 10460 million |
| CAGR | 8.7% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Argenta Group (Klifovet), Charles River Laboratories, Clinvet, IDEXX Laboratories, Inotiv, Knoell – Triveritas, Labcorp Drug Development, Oncovet Clinical Research (OCR), Veterinary Research Management, Vetio, VETSPIN, Zoetis-Nexvet |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
