What is Global Live Attenuated Influenza Vaccine (LAIV) Market?
The Global Live Attenuated Influenza Vaccine (LAIV) Market is a significant segment within the broader influenza vaccine market. Live Attenuated Influenza Vaccines are designed to protect against the flu by using a weakened form of the virus that cannot cause illness. These vaccines are typically administered via a nasal spray, making them a needle-free option that is particularly appealing for children and those who are needle-averse. The global market for LAIV is driven by the increasing awareness of flu prevention, government initiatives to promote vaccination, and the rising incidence of influenza outbreaks. The market encompasses various regions, including North America, Europe, Asia-Pacific, and Latin America, each with its own regulatory landscape and market dynamics. The demand for LAIV is also influenced by factors such as the effectiveness of the vaccine, ease of administration, and public health policies. As the world continues to grapple with seasonal flu and potential pandemics, the importance of effective vaccination strategies like LAIV cannot be overstated.
Trivalent Flu Vaccine, Quadrivalent Flu Vaccine in the Global Live Attenuated Influenza Vaccine (LAIV) Market:
The Trivalent Flu Vaccine and Quadrivalent Flu Vaccine are two types of influenza vaccines that play crucial roles in the Global Live Attenuated Influenza Vaccine (LAIV) Market. The Trivalent Flu Vaccine is designed to protect against three different flu viruses: two influenza A viruses and one influenza B virus. This type of vaccine has been traditionally used and has shown effectiveness in preventing flu-related illnesses. However, the limitation of the Trivalent Flu Vaccine is that it does not cover all circulating strains of the influenza B virus, which can lead to reduced effectiveness in some flu seasons. On the other hand, the Quadrivalent Flu Vaccine is designed to protect against four different flu viruses: two influenza A viruses and two influenza B viruses. This broader coverage makes the Quadrivalent Flu Vaccine a more comprehensive option, as it includes an additional B virus strain that the Trivalent version does not cover. The inclusion of the extra B virus strain in the Quadrivalent vaccine aims to provide better protection against the flu, especially in seasons where multiple B virus strains are circulating. The choice between Trivalent and Quadrivalent vaccines often depends on factors such as age, health status, and specific recommendations from health authorities. Both types of vaccines are available in the form of Live Attenuated Influenza Vaccines (LAIV), which are administered via a nasal spray. This method of administration is particularly advantageous for children and individuals who are afraid of needles. The ease of use and needle-free administration make LAIVs a popular choice in pediatric and public health settings. Additionally, the effectiveness of these vaccines in preventing flu-related complications and hospitalizations further underscores their importance in the global market. As the flu virus continues to evolve, the development and distribution of both Trivalent and Quadrivalent Flu Vaccines remain critical components of global influenza prevention strategies.
Hospital, Clinic, Public Health Agency, Other in the Global Live Attenuated Influenza Vaccine (LAIV) Market:
The usage of Global Live Attenuated Influenza Vaccine (LAIV) spans various settings, including hospitals, clinics, public health agencies, and other healthcare facilities. In hospitals, LAIVs are often administered to healthcare workers and patients to reduce the risk of flu outbreaks within the facility. Hospitals are high-risk environments where the spread of infectious diseases can have severe consequences, making flu vaccination a critical preventive measure. By vaccinating healthcare workers, hospitals aim to protect both staff and patients from influenza, thereby maintaining a healthier environment and reducing absenteeism among healthcare providers. In clinics, LAIVs are commonly used for routine immunization, especially during the flu season. Clinics serve as accessible points of care for the general population, making them ideal locations for administering flu vaccines. The nasal spray form of LAIV is particularly popular in pediatric clinics, as it offers a needle-free option that is less intimidating for children. Public health agencies play a crucial role in the distribution and administration of LAIVs. These agencies often conduct vaccination campaigns and outreach programs to ensure that high-risk populations, such as the elderly, young children, and individuals with chronic health conditions, receive their flu vaccines. Public health agencies also collaborate with schools, community centers, and other organizations to facilitate mass vaccination efforts. In addition to hospitals, clinics, and public health agencies, other settings such as pharmacies, workplaces, and long-term care facilities also utilize LAIVs. Pharmacies provide convenient locations for individuals to receive their flu vaccines without needing to schedule a doctor's appointment. Workplaces may offer flu vaccination programs to protect employees and reduce the impact of flu-related absenteeism. Long-term care facilities, which house vulnerable populations such as the elderly and individuals with chronic illnesses, prioritize flu vaccination to prevent outbreaks and protect residents. Overall, the widespread use of LAIVs across various healthcare settings highlights their importance in preventing influenza and promoting public health.
Global Live Attenuated Influenza Vaccine (LAIV) Market Outlook:
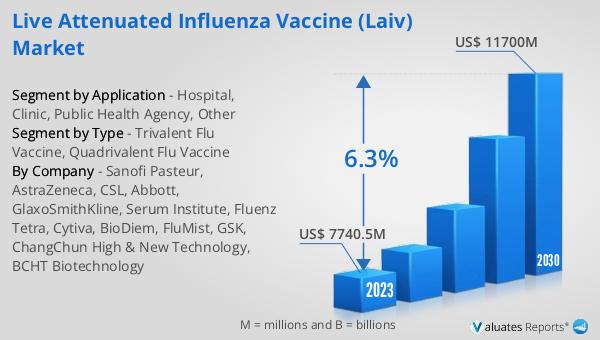
The global market for Live Attenuated Influenza Vaccine (LAIV) was valued at approximately $7.74 billion in 2023. Projections indicate that this market is expected to grow significantly, reaching around $11.7 billion by the year 2030. This growth is anticipated to occur at a compound annual growth rate (CAGR) of 6.3% during the forecast period from 2024 to 2030. The increasing market value reflects the rising demand for effective flu prevention methods, driven by factors such as heightened awareness of influenza risks, government vaccination initiatives, and the ongoing threat of seasonal flu outbreaks. The needle-free administration of LAIVs, coupled with their effectiveness in preventing flu-related complications, makes them a preferred choice in various healthcare settings. As the global population continues to prioritize health and wellness, the demand for reliable and accessible flu vaccines like LAIV is expected to sustain this upward market trend.
| Report Metric | Details |
| Report Name | Live Attenuated Influenza Vaccine (LAIV) Market |
| Accounted market size in 2023 | US$ 7740.5 million |
| Forecasted market size in 2030 | US$ 11700 million |
| CAGR | 6.3% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Sanofi Pasteur, AstraZeneca, CSL, Abbott, GlaxoSmithKline, Serum Institute, Fluenz Tetra, Cytiva, BioDiem, FluMist, GSK, ChangChun High & New Technology, BCHT Biotechnology |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
