What is Global Genetic Toxicology Market?
The Global Genetic Toxicology Market is a specialized sector within the broader field of toxicology that focuses on studying the effects of chemical, physical, and biological agents on genetic material. This market encompasses a range of products and services designed to detect and evaluate genetic damage, which can lead to mutations, cancer, and other genetic disorders. The primary goal of genetic toxicology is to identify potential genotoxic agents and assess their risk to human health and the environment. This market includes various testing methods, such as in vitro and in vivo assays, to evaluate the genotoxic potential of substances. The demand for genetic toxicology testing is driven by regulatory requirements, the need for safer pharmaceuticals, and the increasing awareness of environmental and occupational hazards. As a result, the Global Genetic Toxicology Market plays a crucial role in ensuring the safety and efficacy of new drugs, chemicals, and other products before they are released to the market.
Genotoxicity Screening Assays, GLP Standard Genotoxicity Assays in the Global Genetic Toxicology Market:
Genotoxicity screening assays are essential tools in the Global Genetic Toxicology Market, used to detect and evaluate the potential genotoxic effects of various substances. These assays are designed to identify DNA damage, mutations, and chromosomal alterations that could lead to cancer or other genetic disorders. One of the most common genotoxicity screening assays is the Ames test, also known as the bacterial reverse mutation assay. This test uses strains of the bacterium Salmonella typhimurium to detect mutations caused by chemical substances. Another widely used assay is the in vitro micronucleus test, which measures the formation of micronuclei in cultured cells as an indicator of chromosomal damage. Additionally, the chromosome aberration test evaluates structural changes in chromosomes, providing further insights into the genotoxic potential of a substance. Good Laboratory Practice (GLP) standard genotoxicity assays are conducted under strict regulatory guidelines to ensure the reliability and reproducibility of the results. These assays are essential for regulatory submissions and are often required by agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). GLP standard assays include the in vivo micronucleus test, which assesses chromosomal damage in bone marrow or peripheral blood cells of animals, and the comet assay, which detects DNA strand breaks in individual cells. The Global Genetic Toxicology Market also includes advanced techniques such as next-generation sequencing (NGS) and high-throughput screening (HTS) to enhance the sensitivity and specificity of genotoxicity testing. These cutting-edge technologies enable the detection of low-frequency mutations and provide a comprehensive assessment of genetic damage. Overall, genotoxicity screening assays and GLP standard genotoxicity assays are critical components of the Global Genetic Toxicology Market, ensuring the safety and efficacy of new drugs, chemicals, and other products.
Mini Ames/Ames, Gene Mutation, In Vitro Micronucleus, Chromosome Aberration, Others in the Global Genetic Toxicology Market:
The Global Genetic Toxicology Market finds extensive usage in various areas, including Mini Ames/Ames, gene mutation, in vitro micronucleus, chromosome aberration, and others. The Mini Ames test, a simplified version of the traditional Ames test, is used to screen for mutagenic potential in a high-throughput format. This assay is particularly useful in the early stages of drug development, allowing researchers to quickly identify compounds with genotoxic potential. The gene mutation assays, such as the mouse lymphoma assay and the HPRT assay, are designed to detect point mutations in specific genes. These assays are crucial for evaluating the mutagenic potential of substances and are often used in regulatory submissions. The in vitro micronucleus test is another widely used assay in the Global Genetic Toxicology Market. This test measures the formation of micronuclei in cultured cells, which are indicative of chromosomal damage. The in vitro micronucleus test is commonly used in the pharmaceutical and chemical industries to assess the genotoxic potential of new compounds. Chromosome aberration assays, which evaluate structural changes in chromosomes, are also an essential part of the Global Genetic Toxicology Market. These assays provide valuable information on the clastogenic potential of substances and are often required by regulatory agencies. Other areas of usage in the Global Genetic Toxicology Market include the comet assay, which detects DNA strand breaks in individual cells, and the sister chromatid exchange (SCE) assay, which measures the exchange of genetic material between sister chromatids. These assays are used to assess the genotoxic potential of a wide range of substances, including pharmaceuticals, chemicals, and environmental pollutants. Overall, the Global Genetic Toxicology Market plays a critical role in ensuring the safety and efficacy of new drugs, chemicals, and other products by providing reliable and comprehensive genotoxicity testing.
Global Genetic Toxicology Market Outlook:
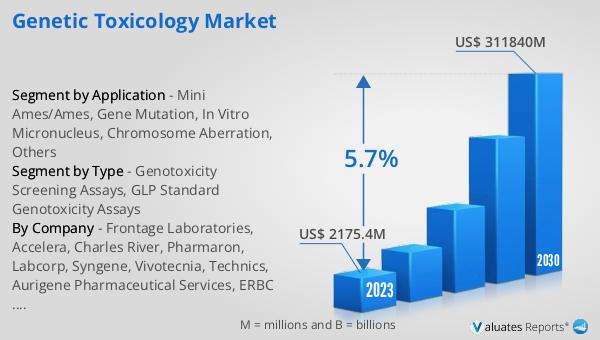
The global Genetic Toxicology market was valued at US$ 2175.4 million in 2023 and is anticipated to reach US$ 311840 million by 2030, witnessing a CAGR of 5.7% during the forecast period 2024-2030. This market outlook highlights the significant growth potential of the Genetic Toxicology market, driven by increasing regulatory requirements, the need for safer pharmaceuticals, and growing awareness of environmental and occupational hazards. The market's valuation in 2023 reflects the substantial demand for genetic toxicology testing, which is expected to continue to rise in the coming years. The projected growth rate of 5.7% CAGR indicates a steady increase in the adoption of genetic toxicology assays and services, driven by advancements in technology and the development of new testing methods. The anticipated market value of US$ 311840 million by 2030 underscores the importance of genetic toxicology in ensuring the safety and efficacy of new drugs, chemicals, and other products. As the market continues to expand, it will play a crucial role in protecting human health and the environment from the potential risks associated with genotoxic agents.
| Report Metric | Details |
| Report Name | Genetic Toxicology Market |
| Accounted market size in 2023 | US$ 2175.4 million |
| Forecasted market size in 2030 | US$ 311840 million |
| CAGR | 5.7% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Frontage Laboratories, Accelera, Charles River, Pharmaron, Labcorp, Syngene, Vivotecnia, Technics, Aurigene Pharmaceutical Services, ERBC Group, NextGen, Cardinal Health, Toxys, Abbott Toxicology, Evotec, EPL |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
