What is Global Live Attenuated Varicella Vaccine Market?
The Global Live Attenuated Varicella Vaccine Market refers to the worldwide industry focused on the development, production, and distribution of vaccines designed to prevent varicella, commonly known as chickenpox. These vaccines are created using a live but weakened form of the varicella-zoster virus, which helps the immune system recognize and combat the virus without causing the disease itself. The market encompasses various stakeholders, including pharmaceutical companies, healthcare providers, and regulatory bodies, all working together to ensure the availability and efficacy of these vaccines. The demand for live attenuated varicella vaccines is driven by the need to reduce the incidence of chickenpox, which can lead to severe complications, especially in immunocompromised individuals and pregnant women. The market is also influenced by factors such as government vaccination programs, public awareness campaigns, and advancements in vaccine technology. As a result, the Global Live Attenuated Varicella Vaccine Market plays a crucial role in public health by providing a reliable means of preventing a common and potentially serious infectious disease.
Monovalent Vaccine, Combination Vaccine in the Global Live Attenuated Varicella Vaccine Market:
Monovalent vaccines and combination vaccines are two primary types of vaccines available in the Global Live Attenuated Varicella Vaccine Market. Monovalent vaccines are designed to immunize against a single pathogen, in this case, the varicella-zoster virus. These vaccines are particularly beneficial for targeting specific diseases and are often used in routine childhood immunization schedules. Monovalent varicella vaccines are typically administered in two doses, with the first dose given to children between 12 and 15 months of age and the second dose between 4 and 6 years of age. This vaccination schedule ensures that children develop strong immunity against chickenpox, reducing the risk of outbreaks and complications associated with the disease. On the other hand, combination vaccines are designed to protect against multiple diseases with a single injection. In the context of the Global Live Attenuated Varicella Vaccine Market, combination vaccines often include the varicella vaccine along with vaccines for other common childhood diseases such as measles, mumps, and rubella (MMR). The MMRV vaccine, for example, combines the measles, mumps, rubella, and varicella vaccines into one shot, simplifying the vaccination process and increasing compliance among parents and healthcare providers. Combination vaccines offer several advantages, including reduced healthcare visits, lower administration costs, and improved vaccination coverage. They are particularly useful in settings where access to healthcare is limited, as they ensure that children receive protection against multiple diseases in a single visit. Both monovalent and combination vaccines undergo rigorous testing and regulatory approval processes to ensure their safety and efficacy. Clinical trials are conducted to evaluate the immune response generated by the vaccines, their ability to prevent disease, and any potential side effects. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) play a crucial role in overseeing these processes and granting approval for the vaccines to be marketed and distributed. The choice between monovalent and combination vaccines depends on various factors, including the target population, healthcare infrastructure, and public health goals. In some cases, monovalent vaccines may be preferred for their targeted approach, while in others, combination vaccines may be favored for their convenience and broader protection. Ultimately, both types of vaccines contribute significantly to the Global Live Attenuated Varicella Vaccine Market by providing effective means of preventing varicella and other infectious diseases.
Kids Injection, Adults Injection in the Global Live Attenuated Varicella Vaccine Market:
The usage of the Global Live Attenuated Varicella Vaccine Market extends to both kids and adults, with specific formulations and administration protocols tailored to each group. For kids, the varicella vaccine is typically administered as an injection in two doses. The first dose is given to children between 12 and 15 months of age, and the second dose is administered between 4 and 6 years of age. This vaccination schedule is designed to provide early and robust immunity against chickenpox, a highly contagious disease that can lead to severe complications such as bacterial infections, pneumonia, and encephalitis. By vaccinating children at a young age, the risk of outbreaks in schools and daycare centers is significantly reduced, contributing to overall public health and safety. In addition to routine childhood immunization, the varicella vaccine is also recommended for older children and adolescents who have not previously been vaccinated or have not had chickenpox. Catch-up vaccination programs are often implemented to ensure that these individuals receive the necessary protection against the disease. For adults, the varicella vaccine is particularly important for those who have never had chickenpox or been vaccinated. Adults who contract chickenpox are at a higher risk of severe complications, including pneumonia, hepatitis, and encephalitis. Pregnant women who contract chickenpox are at risk of serious complications for both themselves and their unborn babies, including congenital varicella syndrome, which can cause birth defects. Therefore, it is crucial for women of childbearing age to be vaccinated before becoming pregnant if they are not already immune. The varicella vaccine is also recommended for healthcare workers, teachers, and other adults who work in environments where they are likely to be exposed to the virus. In these settings, vaccination helps prevent the spread of chickenpox to vulnerable populations, such as immunocompromised individuals and infants who are too young to be vaccinated. The administration of the varicella vaccine to adults typically involves two doses, given 4 to 8 weeks apart. The vaccine is generally well-tolerated, with mild side effects such as soreness at the injection site, fever, and rash being the most common. Serious side effects are rare, but as with any medical intervention, it is important for individuals to discuss their medical history and any potential contraindications with their healthcare provider before receiving the vaccine. Overall, the Global Live Attenuated Varicella Vaccine Market plays a vital role in protecting both kids and adults from chickenpox, reducing the incidence of the disease and its associated complications. By ensuring widespread vaccination coverage, public health authorities can effectively control and prevent outbreaks, safeguarding the health and well-being of communities worldwide.
Global Live Attenuated Varicella Vaccine Market Outlook:
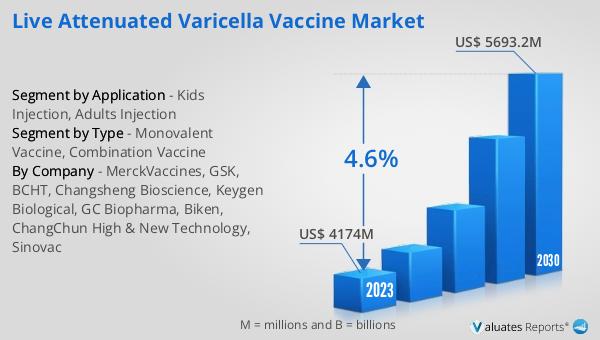
The global Live Attenuated Varicella Vaccine market was valued at US$ 4174 million in 2023 and is anticipated to reach US$ 5693.2 million by 2030, witnessing a CAGR of 4.6% during the forecast period 2024-2030. The global pharmaceutical market is valued at 1475 billion USD in 2022, growing at a CAGR of 5% over the next six years. In comparison, the chemical drug market is estimated to increase from 1005 billion USD in 2018 to 1094 billion USD in 2022. This data highlights the significant growth and potential of the Live Attenuated Varicella Vaccine market within the broader pharmaceutical industry. The increasing demand for vaccines, driven by public health initiatives and advancements in vaccine technology, is expected to contribute to the market's expansion. As the pharmaceutical industry continues to grow, the Live Attenuated Varicella Vaccine market is poised to play a crucial role in preventing varicella and improving global health outcomes.
| Report Metric | Details |
| Report Name | Live Attenuated Varicella Vaccine Market |
| Accounted market size in 2023 | US$ 4174 million |
| Forecasted market size in 2030 | US$ 5693.2 million |
| CAGR | 4.6% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | MerckVaccines, GSK, BCHT, Changsheng Bioscience, Keygen Biological, GC Biopharma, Biken, ChangChun High & New Technology, Sinovac |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
