What is Global Diabetic Macular Edema Drug Market?
The Global Diabetic Macular Edema Drug Market is a specialized segment within the broader pharmaceutical industry, focusing on treatments for diabetic macular edema (DME), a condition that affects the eyes of individuals with diabetes. DME occurs when fluid accumulates in the macula, the central part of the retina, leading to vision impairment or loss. The market for these drugs is driven by the increasing prevalence of diabetes worldwide, which in turn raises the incidence of DME. As the global population ages and lifestyles change, the number of people affected by diabetes and its complications, such as DME, is expected to rise. This market includes a variety of treatment options, such as anti-VEGF (vascular endothelial growth factor) therapies, corticosteroids, and other emerging treatments. Pharmaceutical companies are investing heavily in research and development to create more effective and safer drugs to manage this condition. The market is characterized by intense competition, with numerous players striving to develop innovative solutions to improve patient outcomes. As awareness of DME and its impact on quality of life grows, the demand for effective treatments is likely to increase, making this a dynamic and evolving market.
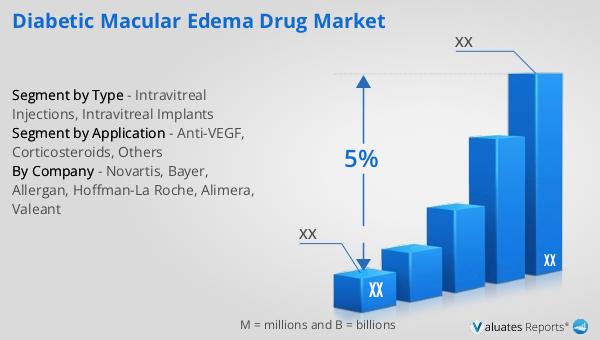
Intravitreal Injections, Intravitreal Implants in the Global Diabetic Macular Edema Drug Market:
Intravitreal injections and intravitreal implants are two critical methods of delivering medication directly into the eye to treat diabetic macular edema (DME). Intravitreal injections involve administering drugs directly into the vitreous humor, the gel-like substance inside the eye. This method ensures that the medication reaches the retina quickly and in high concentrations, which is crucial for treating conditions like DME. The most common drugs delivered via intravitreal injections are anti-VEGF agents and corticosteroids. Anti-VEGF drugs work by inhibiting the growth of abnormal blood vessels and reducing fluid leakage, which are key contributors to DME. Corticosteroids, on the other hand, help reduce inflammation and swelling in the retina. The procedure for intravitreal injections is relatively quick and is usually performed in an outpatient setting. Patients may experience mild discomfort during the injection, but serious complications are rare when performed by a skilled ophthalmologist. Intravitreal implants, on the other hand, are tiny devices placed inside the eye that slowly release medication over an extended period. These implants are particularly beneficial for patients who require long-term treatment, as they reduce the need for frequent injections. The implants can deliver a steady dose of medication, ensuring consistent therapeutic effects. One of the most well-known intravitreal implants is the dexamethasone implant, which releases corticosteroids to manage inflammation and edema. The choice between intravitreal injections and implants depends on various factors, including the severity of the condition, patient preference, and the specific drug being used. Both methods have their advantages and limitations, and the decision is usually made in consultation with an ophthalmologist. As research continues, new drugs and delivery methods are being developed to improve the efficacy and safety of treatments for DME. The Global Diabetic Macular Edema Drug Market is witnessing significant advancements in this area, with companies exploring novel drug formulations and delivery systems to enhance patient outcomes. The development of biodegradable implants and sustained-release formulations are some of the innovations being pursued to address the challenges associated with current treatment options. These advancements aim to improve patient compliance, reduce the frequency of treatments, and minimize side effects. As the market evolves, the focus remains on providing effective and convenient solutions for managing DME, ultimately improving the quality of life for patients affected by this condition.
Anti-VEGF, Corticosteroids, Others in the Global Diabetic Macular Edema Drug Market:
The Global Diabetic Macular Edema Drug Market encompasses a range of treatment options, with anti-VEGF therapies, corticosteroids, and other emerging treatments playing significant roles. Anti-VEGF therapies are among the most widely used treatments for DME. These drugs work by inhibiting the action of vascular endothelial growth factor (VEGF), a protein that promotes the growth of abnormal blood vessels and increases vascular permeability. By blocking VEGF, these drugs help reduce fluid leakage and swelling in the retina, thereby improving vision. Common anti-VEGF drugs used in the treatment of DME include ranibizumab, aflibercept, and bevacizumab. These medications are typically administered via intravitreal injections, and their efficacy in improving visual acuity and reducing retinal thickness has been well-documented in clinical trials. Corticosteroids are another important class of drugs used in the management of DME. These medications help reduce inflammation and edema in the retina, which are key contributors to vision impairment in DME patients. Corticosteroids can be administered as intravitreal injections or implants, with the latter offering the advantage of sustained drug release over an extended period. Dexamethasone and fluocinolone acetonide are examples of corticosteroids used in the treatment of DME. While corticosteroids are effective in reducing retinal swelling, they are associated with potential side effects such as increased intraocular pressure and cataract formation, which need to be carefully monitored. In addition to anti-VEGF therapies and corticosteroids, the Global Diabetic Macular Edema Drug Market is witnessing the development of other innovative treatments. These include novel drug formulations, combination therapies, and gene therapies aimed at providing more effective and personalized treatment options for DME patients. Combination therapies, which involve the use of multiple drugs with different mechanisms of action, are being explored to enhance treatment efficacy and reduce the frequency of injections. Gene therapies, although still in the experimental stage, hold promise for providing long-term solutions by targeting the underlying genetic causes of DME. As the market continues to evolve, the focus remains on improving patient outcomes and quality of life through the development of safer and more effective treatments. The increasing understanding of the pathophysiology of DME and advancements in drug delivery technologies are driving innovation in this field, offering hope for better management of this challenging condition.
Global Diabetic Macular Edema Drug Market Outlook:
The outlook for the Global Diabetic Macular Edema Drug Market can be contextualized within the broader pharmaceutical industry trends. In 2022, the global pharmaceutical market was valued at approximately 1,475 billion USD, with an anticipated compound annual growth rate (CAGR) of 5% over the next six years. This growth is indicative of the increasing demand for pharmaceutical products, driven by factors such as the rising prevalence of chronic diseases, advancements in drug development, and expanding healthcare access in emerging markets. Within this context, the chemical drug market, a significant component of the pharmaceutical industry, has shown substantial growth. From 2018 to 2022, the chemical drug market expanded from 1,005 billion USD to 1,094 billion USD. This growth reflects the ongoing innovation and development of new chemical entities and formulations to address a wide range of medical conditions, including diabetic macular edema. The increasing focus on personalized medicine and targeted therapies is also contributing to the growth of the chemical drug market. As pharmaceutical companies continue to invest in research and development, the introduction of novel drugs and treatment modalities is expected to drive further growth in the market. The Global Diabetic Macular Edema Drug Market, as a part of this broader industry, is poised to benefit from these trends, with ongoing advancements in drug delivery technologies and the development of more effective treatments for DME. The market's growth is supported by the increasing prevalence of diabetes and its complications, as well as the growing awareness of the impact of DME on patients' quality of life. As the market evolves, the focus remains on improving patient outcomes through the development of innovative and effective treatment options.
| Report Metric | Details |
| Report Name | Diabetic Macular Edema Drug Market |
| CAGR | 5% |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Novartis, Bayer, Allergan, Hoffman-La Roche, Alimera, Valeant |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |