What is Global Actinic (Solar) Keratosis Treatment Market?
The Global Actinic (Solar) Keratosis Treatment Market refers to the industry focused on developing and providing treatments for actinic keratosis, a skin condition caused by prolonged exposure to ultraviolet (UV) rays from the sun. This condition is characterized by rough, scaly patches on the skin, primarily affecting areas frequently exposed to the sun, such as the face, ears, neck, scalp, and hands. Actinic keratosis is considered a precancerous condition, as it can potentially progress to squamous cell carcinoma, a type of skin cancer, if left untreated. The market for actinic keratosis treatment includes a variety of therapeutic options, such as topical medications, cryotherapy, photodynamic therapy, and surgical procedures. The increasing awareness of skin cancer risks, coupled with the growing prevalence of actinic keratosis due to rising sun exposure and aging populations, drives the demand for effective treatments. Additionally, advancements in dermatological research and technology contribute to the development of innovative therapies, further expanding the market. As a result, the Global Actinic (Solar) Keratosis Treatment Market plays a crucial role in addressing the healthcare needs of individuals affected by this common skin condition.
854-A, ACT-01, AD-17137, Celecoxib, DFD-08, GDC-695, Others in the Global Actinic (Solar) Keratosis Treatment Market:
The Global Actinic (Solar) Keratosis Treatment Market encompasses a range of therapeutic agents, including 854-A, ACT-01, AD-17137, Celecoxib, DFD-08, GDC-695, and others, each contributing uniquely to the management of actinic keratosis. 854-A is a promising compound under investigation, known for its potential to target specific pathways involved in the development of actinic keratosis lesions. This compound aims to provide a targeted approach, minimizing damage to surrounding healthy tissues. ACT-01, another innovative treatment, focuses on modulating the immune response to enhance the body's ability to combat precancerous cells. By boosting the immune system's activity, ACT-01 offers a novel mechanism for addressing actinic keratosis. AD-17137 is a topical formulation designed to penetrate the skin effectively, delivering active ingredients directly to the affected areas. This targeted delivery system enhances the efficacy of the treatment while reducing systemic side effects. Celecoxib, a well-known nonsteroidal anti-inflammatory drug (NSAID), has shown potential in reducing the risk of actinic keratosis progression. Its anti-inflammatory properties help alleviate the symptoms associated with the condition, providing relief to patients. DFD-08 is a topical gel formulation that combines multiple active ingredients to address actinic keratosis comprehensively. This multi-faceted approach targets different aspects of the condition, offering a holistic treatment option. GDC-695, a novel agent, is being explored for its ability to inhibit specific enzymes involved in the proliferation of precancerous cells. By targeting these enzymes, GDC-695 aims to prevent the progression of actinic keratosis to more severe forms of skin cancer. In addition to these specific treatments, the market also includes other therapeutic options, such as cryotherapy, photodynamic therapy, and surgical interventions. Cryotherapy involves freezing the affected skin cells, effectively destroying them and preventing further progression. Photodynamic therapy utilizes light-activated compounds to selectively target and destroy precancerous cells, offering a non-invasive treatment option. Surgical procedures, such as curettage and excision, are employed for more advanced cases, where lesions are removed to prevent cancerous transformation. The diverse range of treatments available in the Global Actinic (Solar) Keratosis Treatment Market reflects the complexity of the condition and the need for tailored approaches to meet individual patient needs. As research continues to advance, these treatments hold the potential to improve patient outcomes and reduce the burden of actinic keratosis on individuals and healthcare systems worldwide.
Hospital, Clinic, Others in the Global Actinic (Solar) Keratosis Treatment Market:
The usage of the Global Actinic (Solar) Keratosis Treatment Market spans various healthcare settings, including hospitals, clinics, and other medical facilities, each playing a vital role in managing this prevalent skin condition. In hospitals, the treatment of actinic keratosis often involves a multidisciplinary approach, where dermatologists, oncologists, and other specialists collaborate to provide comprehensive care. Hospitals are equipped with advanced diagnostic tools and treatment technologies, enabling precise assessment and intervention for patients with actinic keratosis. In this setting, patients may undergo procedures such as cryotherapy, photodynamic therapy, or surgical excision, depending on the severity and extent of their condition. The hospital environment also facilitates the management of complex cases, where patients may require a combination of treatments to achieve optimal outcomes. Clinics, on the other hand, offer a more accessible and convenient option for patients seeking treatment for actinic keratosis. Dermatology clinics, in particular, specialize in skin conditions and provide targeted therapies tailored to individual patient needs. In these settings, patients can receive topical treatments, such as AD-17137 or DFD-08, which are applied directly to the affected areas. Clinics also offer follow-up care and monitoring, ensuring that patients receive ongoing support and adjustments to their treatment plans as needed. The personalized approach in clinics allows for early intervention and management of actinic keratosis, reducing the risk of progression to more severe forms of skin cancer. Beyond hospitals and clinics, other healthcare facilities, such as outpatient centers and specialized dermatology practices, contribute to the treatment landscape for actinic keratosis. These facilities often focus on preventive care and education, raising awareness about the importance of sun protection and regular skin checks. By promoting early detection and intervention, these settings play a crucial role in reducing the incidence and impact of actinic keratosis. Additionally, telemedicine platforms have emerged as valuable tools in the management of actinic keratosis, allowing patients to consult with healthcare providers remotely and receive guidance on treatment options. This approach enhances accessibility to care, particularly for individuals in remote or underserved areas. Overall, the Global Actinic (Solar) Keratosis Treatment Market's presence in hospitals, clinics, and other healthcare settings underscores the importance of a comprehensive and coordinated approach to managing this common skin condition. By leveraging the strengths of each setting, healthcare providers can deliver effective and timely interventions, improving patient outcomes and quality of life.
Global Actinic (Solar) Keratosis Treatment Market Outlook:
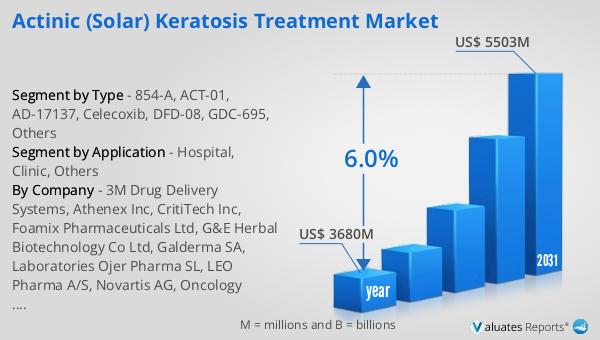
In 2024, the global market for Actinic Solar Keratosis Treatment was valued at approximately $3.68 billion. It is anticipated to grow significantly, reaching an estimated size of $5.503 billion by 2031, with a compound annual growth rate (CAGR) of 6.0% throughout the forecast period. In the broader context, the global pharmaceutical market was valued at $1.475 trillion in 2022, with an expected growth rate of 5% over the next six years. Comparatively, the chemical drug market has shown an increase from $1.005 trillion in 2018 to $1.094 trillion in 2022. This data highlights the robust growth potential within the Actinic Solar Keratosis Treatment Market, driven by increasing awareness of skin health and the rising prevalence of actinic keratosis. The market's expansion is supported by advancements in dermatological research and the development of innovative treatment options. As the demand for effective therapies continues to rise, the Actinic Solar Keratosis Treatment Market is poised to play a crucial role in addressing the healthcare needs of individuals affected by this condition. The projected growth reflects the market's capacity to adapt to evolving patient needs and technological advancements, ensuring improved outcomes for those seeking treatment for actinic keratosis.
| Report Metric | Details |
| Report Name | Actinic (Solar) Keratosis Treatment Market |
| Accounted market size in year | US$ 3680 million |
| Forecasted market size in 2031 | US$ 5503 million |
| CAGR | 6.0% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | 3M Drug Delivery Systems, Athenex Inc, CritiTech Inc, Foamix Pharmaceuticals Ltd, G&E Herbal Biotechnology Co Ltd, Galderma SA, Laboratories Ojer Pharma SL, LEO Pharma A/S, Novartis AG, Oncology Research International Ltd, Promius Pharma LLC, Valeant Pharmaceuticals International Inc, Vectura Group Plc |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
