What is Global Healthcare Supply Chain Management Software Market?
The Global Healthcare Supply Chain Management Software Market is a crucial component in the healthcare industry, designed to streamline and optimize the complex processes involved in the supply chain. This software helps manage the flow of medical goods and services from manufacturers to end-users, ensuring that the right products are delivered at the right time and place. It encompasses various functions such as procurement, inventory management, order processing, and logistics. By integrating these processes, the software enhances efficiency, reduces costs, and minimizes errors, ultimately improving patient care. The market for this software is driven by the increasing demand for efficient healthcare services, the need for cost reduction, and the growing adoption of advanced technologies. As healthcare systems worldwide face challenges like rising costs and regulatory pressures, the adoption of supply chain management software becomes essential for maintaining operational efficiency and ensuring the timely delivery of medical products. This market is expected to grow as healthcare providers and organizations continue to seek innovative solutions to enhance their supply chain operations.
Cloud Based, On Permise in the Global Healthcare Supply Chain Management Software Market:
Cloud-based and on-premise solutions are two primary deployment models in the Global Healthcare Supply Chain Management Software Market, each offering distinct advantages and considerations. Cloud-based solutions are hosted on remote servers and accessed via the internet, providing flexibility and scalability. They allow healthcare organizations to access the software from anywhere, facilitating real-time data sharing and collaboration among stakeholders. This model is particularly beneficial for organizations with multiple locations or those looking to reduce IT infrastructure costs. Cloud-based solutions often come with automatic updates and maintenance, ensuring that users always have access to the latest features and security enhancements. However, concerns about data security and privacy can be a drawback, as sensitive healthcare information is stored off-site. On the other hand, on-premise solutions are installed locally on an organization's servers, offering greater control over data and security. This model is preferred by organizations with stringent data protection requirements or those operating in regions with strict regulatory compliance. On-premise solutions can be customized to meet specific organizational needs, but they require significant upfront investment in hardware and ongoing maintenance. Additionally, scalability can be a challenge, as expanding capacity often involves purchasing additional hardware. Both deployment models have their place in the healthcare supply chain management landscape, and the choice between them depends on factors such as organizational size, budget, regulatory requirements, and IT capabilities. As technology continues to evolve, hybrid models that combine elements of both cloud-based and on-premise solutions are also emerging, offering a balance between flexibility and control. These hybrid solutions allow organizations to leverage the benefits of cloud computing while maintaining critical data on-premise, providing a tailored approach to supply chain management. Ultimately, the decision between cloud-based and on-premise solutions should be guided by a thorough assessment of an organization's specific needs and strategic goals.
Manufacturers, Providers, Distributors in the Global Healthcare Supply Chain Management Software Market:
The usage of Global Healthcare Supply Chain Management Software Market spans across various sectors, including manufacturers, providers, and distributors, each benefiting from its capabilities in unique ways. For manufacturers, the software plays a pivotal role in managing production schedules, tracking raw materials, and ensuring timely delivery of finished products. It enables manufacturers to optimize their supply chain operations by providing real-time visibility into inventory levels, demand forecasts, and supplier performance. This visibility helps manufacturers reduce lead times, minimize stockouts, and improve overall production efficiency. Additionally, the software facilitates compliance with regulatory standards by maintaining accurate records and documentation, which is crucial in the highly regulated healthcare industry. For healthcare providers, such as hospitals and clinics, supply chain management software is essential for managing inventory, reducing waste, and ensuring the availability of critical medical supplies. Providers can use the software to track usage patterns, automate reordering processes, and manage supplier relationships, leading to cost savings and improved patient care. The software also supports providers in managing complex logistics, such as coordinating deliveries of time-sensitive products like pharmaceuticals and medical devices. For distributors, the software enhances operational efficiency by streamlining order processing, optimizing warehouse management, and improving transportation logistics. Distributors can leverage the software to manage large volumes of products, track shipments in real-time, and ensure timely delivery to healthcare providers. The software's analytics capabilities enable distributors to identify trends, forecast demand, and make data-driven decisions, ultimately enhancing customer satisfaction and competitive advantage. Overall, the Global Healthcare Supply Chain Management Software Market provides a comprehensive solution for managing the intricate processes involved in the healthcare supply chain, benefiting manufacturers, providers, and distributors alike.
Global Healthcare Supply Chain Management Software Market Outlook:
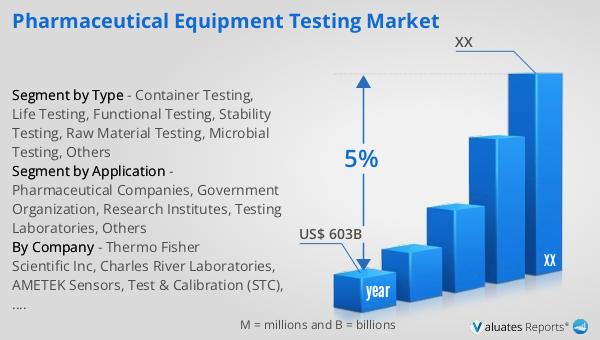
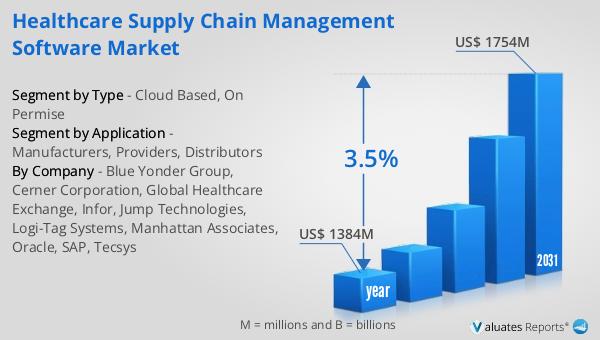
The global market for Healthcare Supply Chain Management Software was valued at $1,384 million in 2024 and is anticipated to grow to a revised size of $1,754 million by 2031, reflecting a compound annual growth rate (CAGR) of 3.5% during the forecast period. This growth is indicative of the increasing demand for efficient supply chain solutions in the healthcare sector, driven by factors such as the need for cost reduction, regulatory compliance, and the adoption of advanced technologies. In parallel, the global market for medical devices is estimated at $603 billion in 2023, with a projected CAGR of 5% over the next six years. This growth in the medical devices market further underscores the importance of effective supply chain management, as the timely delivery and availability of medical devices are critical to healthcare operations. The synergy between the growth of the healthcare supply chain management software market and the medical devices market highlights the interconnected nature of the healthcare industry, where advancements in one area can drive improvements in another. As healthcare organizations continue to navigate the complexities of supply chain management, the adoption of innovative software solutions will be key to achieving operational efficiency and enhancing patient care.
| Report Metric | Details |
| Report Name | Healthcare Supply Chain Management Software Market |
| Accounted market size in year | US$ 1384 million |
| Forecasted market size in 2031 | US$ 1754 million |
| CAGR | 3.5% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Blue Yonder Group, Cerner Corporation, Global Healthcare Exchange, Infor, Jump Technologies, Logi-Tag Systems, Manhattan Associates, Oracle, SAP, Tecsys |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |