What is Pyrazolone - Global Market?
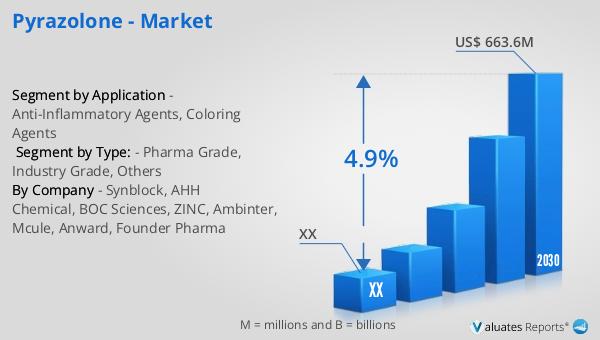
Pyrazolone is a chemical compound that serves as a derivative of pyrazole, characterized by the presence of an additional keto oxygen group. This compound has garnered significant attention in the global market due to its versatile applications across various industries. As of 2023, the global market for Pyrazolone was valued at approximately US$ 476 million. It is projected to grow to a revised size of US$ 663.6 million by 2030, reflecting a compound annual growth rate (CAGR) of 4.9% during the forecast period from 2024 to 2030. This growth trajectory highlights the increasing demand and utilization of Pyrazolone in diverse sectors. In the broader context, the global pharmaceutical market was valued at 1475 billion USD in 2022, with an anticipated growth rate of 5% over the next six years. Comparatively, the chemical drug market has shown an upward trend, expanding from 1005 billion USD in 2018 to 1094 billion USD in 2022. These figures underscore the dynamic nature of the pharmaceutical and chemical industries, within which Pyrazolone plays a crucial role. The compound's unique properties and applications continue to drive its market expansion, making it a significant player in the global chemical landscape.
Pharma Grade, Industry Grade, Others in the Pyrazolone - Global Market:
In the Pyrazolone global market, the compound is categorized into different grades based on its purity and intended use, namely Pharma Grade, Industry Grade, and Others. Pharma Grade Pyrazolone is primarily used in the pharmaceutical industry due to its high purity and stringent quality standards. This grade is essential for the production of various medications, particularly those that require precise chemical compositions to ensure efficacy and safety. The pharmaceutical industry relies heavily on Pharma Grade Pyrazolone for the synthesis of anti-inflammatory drugs, analgesics, and other therapeutic agents. Its role in drug formulation is critical, as it contributes to the development of medications that alleviate pain and inflammation, thereby improving patient outcomes. On the other hand, Industry Grade Pyrazolone is utilized in non-pharmaceutical applications, where the purity requirements are less stringent. This grade finds its use in the manufacturing of dyes, pigments, and other chemical products. The versatility of Industry Grade Pyrazolone makes it a valuable component in the production of coloring agents, which are used in textiles, plastics, and other materials. Its ability to impart vibrant colors and enhance product aesthetics is a key factor driving its demand in the industrial sector. Additionally, the "Others" category encompasses Pyrazolone grades that are used in niche applications or emerging markets. These may include specialized chemical processes, research and development activities, or innovative product formulations. The adaptability of Pyrazolone to various applications underscores its significance in the global market, as it caters to a wide range of industries with diverse needs. The growth of the Pyrazolone market is fueled by its multifaceted applications and the increasing demand for high-quality chemical compounds across different sectors. As industries continue to evolve and innovate, the role of Pyrazolone in facilitating these advancements remains pivotal. Its contribution to both pharmaceutical and industrial applications highlights its importance as a versatile and indispensable chemical compound in the global market.
Anti-Inflammatory Agents, Coloring Agents in the Pyrazolone - Global Market:
Pyrazolone's usage in the global market extends to several key areas, notably as anti-inflammatory agents and coloring agents. As an anti-inflammatory agent, Pyrazolone plays a crucial role in the pharmaceutical industry. It is a core component in the formulation of non-steroidal anti-inflammatory drugs (NSAIDs), which are widely used to treat conditions such as arthritis, muscle pain, and other inflammatory disorders. The compound's ability to inhibit the production of prostaglandins, which are chemicals responsible for inflammation and pain, makes it an effective therapeutic agent. Patients benefit from Pyrazolone-based medications as they provide relief from pain and inflammation, improving their quality of life. The demand for such medications continues to rise, driven by an aging population and the increasing prevalence of chronic inflammatory conditions. In addition to its pharmaceutical applications, Pyrazolone is also utilized as a coloring agent in various industries. Its chemical properties allow it to produce vibrant and stable colors, making it an ideal choice for the production of dyes and pigments. These coloring agents are extensively used in the textile industry to dye fabrics, in the plastics industry to color products, and in the printing industry for inks. The versatility of Pyrazolone as a coloring agent is evident in its ability to produce a wide range of colors, from bright and bold to subtle and muted tones. This adaptability makes it a valuable asset in industries where color quality and consistency are paramount. The global market for Pyrazolone as a coloring agent is driven by the demand for high-quality, durable, and aesthetically pleasing products. As consumer preferences continue to evolve, the need for innovative and diverse coloring solutions remains strong. Pyrazolone's dual role as an anti-inflammatory and coloring agent highlights its significance in the global market. Its ability to address both health-related and aesthetic needs underscores its versatility and importance as a chemical compound. The continued growth and development of the Pyrazolone market are indicative of its enduring relevance and potential for future applications.
Pyrazolone - Global Market Outlook:
English: #PyrazoloneMarket #PharmaGrade #IndustryGrade #ChemicalIndustry #AntiInflammatory #ColoringAgents #GlobalMarket #Pharmaceuticals #ChemicalCompounds #MarketGrowth
| Report Metric | Details |
| Report Name | Pyrazolone - Market |
| Forecasted market size in 2030 | US$ 663.6 million |
| CAGR | 4.9% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Synblock, AHH Chemical, BOC Sciences, ZINC, Ambinter, Mcule, Anward, Founder Pharma |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |