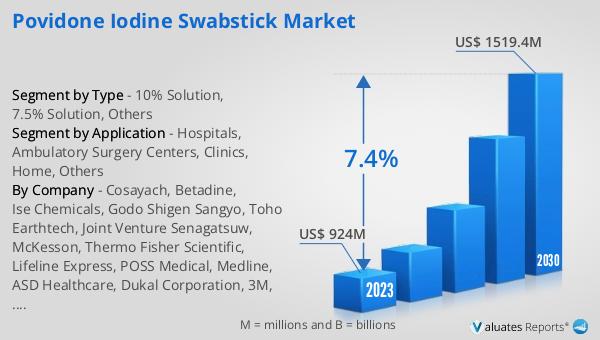
What is Global Zinc Oxide Adhesive Plaster Market?
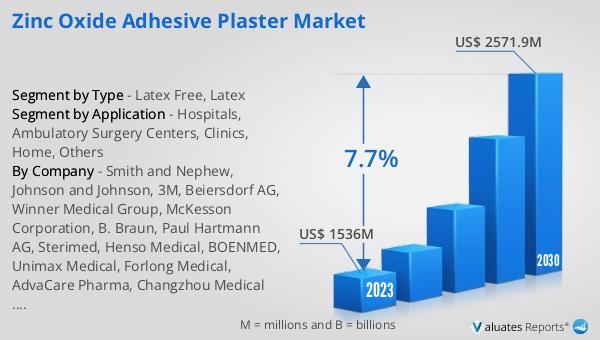
The Global Zinc Oxide Adhesive Plaster Market is a specialized segment within the broader medical supplies industry, focusing on the production and distribution of adhesive plasters that are coated with zinc oxide. This unique composition of the plaster makes it highly beneficial for medical and healthcare applications, particularly because zinc oxide is known for its antiseptic and healing properties. These plasters are extensively used to secure dressings on wounds, cuts, and other skin injuries, providing not only protection but also promoting faster healing. The market encompasses a wide range of products, including different sizes, types, and packaging options to cater to various medical needs and preferences. As of 2023, the market has been valued at US$ 1536 million, showcasing the significant demand and reliance on these plasters in healthcare settings. With an anticipated compound annual growth rate (CAGR) of 7.7%, the market is expected to grow substantially, reaching an estimated value of US$ 2571.9 million by 2030. This growth is reflective of the increasing global demand for effective wound care solutions and the recognized benefits of zinc oxide in wound management and healing.
Latex Free, Latex in the Global Zinc Oxide Adhesive Plaster Market:
Diving deeper into the Global Zinc Oxide Adhesive Plaster Market, it's essential to understand the distinction and significance of Latex Free and Latex-based products within this niche. Latex, a natural rubber substance, is commonly used in various medical supplies, including adhesive plasters, for its elasticity, flexibility, and durability. However, latex can cause allergic reactions in some individuals, ranging from mild skin irritations to severe anaphylactic reactions. This has led to the development and increased demand for Latex Free zinc oxide adhesive plasters, which are made from synthetic or alternative materials that mimic the properties of latex without the associated allergy risks. These Latex Free options provide a safer choice for individuals with latex allergies or sensitivities, ensuring that the benefits of zinc oxide adhesive plasters can be accessed by a broader population without compromising health and safety. On the other hand, Latex-based zinc oxide adhesive plasters continue to be popular for their high performance and cost-effectiveness, making them suitable for various medical, athletic, and personal care applications. The market for both Latex Free and Latex-based zinc oxide adhesive plasters is driven by factors such as the rising awareness of latex allergies, advancements in material science, and the growing demand for effective wound care solutions across healthcare settings. As the market evolves, the development of innovative, hypoallergenic, and more effective adhesive plasters is expected to further propel the growth of this segment, catering to the diverse needs and preferences of consumers worldwide.
Hospitals, Ambulatory Surgery Centers, Clinics, Home, Others in the Global Zinc Oxide Adhesive Plaster Market:
The usage of Global Zinc Oxide Adhesive Plaster Market spans across various healthcare settings, including Hospitals, Ambulatory Surgery Centers, Clinics, Home care, and Others, highlighting its versatility and essential role in wound management and care. In hospitals, these adhesive plasters are widely used in emergency rooms, wards, and surgical units for securing dressings on wounds, supporting IV lines, and even in surgical procedures as a gentle yet effective means to protect and heal the skin. Ambulatory Surgery Centers also rely on zinc oxide adhesive plasters for post-operative care, ensuring wounds are protected and heal properly without the risk of infection. Clinics, serving a broad spectrum of healthcare needs, utilize these plasters for routine wound care, minor surgical procedures, and as part of the standard first-aid kits. The home care segment has seen a significant increase in the use of zinc oxide adhesive plasters as well, with individuals and families using them for everyday cuts, scrapes, and injuries, appreciating their ease of use, effectiveness, and the added benefit of zinc oxide's healing properties. Other areas, including sports medicine, military, and schools, also make extensive use of these plasters for immediate wound care and protection. This widespread usage underscores the importance of zinc oxide adhesive plasters in promoting healing, preventing infection, and providing care across different settings, making them a staple in medical supplies and personal care kits globally.
Global Zinc Oxide Adhesive Plaster Market Outlook:
The market outlook for the Global Zinc Oxide Adhesive Plaster Market presents a promising future, with the market's value standing at US$ 1536 million as of 2023 and an expected surge to US$ 2571.9 million by 2030, marking a CAGR of 7.7% throughout the forecast period of 2024 to 2030. This growth trajectory is indicative of the increasing reliance on and demand for zinc oxide adhesive plasters in the medical and healthcare sectors. The broader medical devices market, within which this segment operates, is also experiencing robust growth, estimated at US$ 603 billion in 2023, with projections to expand at a CAGR of 5% over the next six years. These figures underscore the significant role that zinc oxide adhesive plasters play in the medical supplies industry, driven by their essential function in wound care, the ongoing advancements in healthcare practices, and the growing awareness of the need for effective and safe wound management solutions. The anticipated growth of the Global Zinc Oxide Adhesive Plaster Market reflects the broader trends in healthcare towards more efficient, patient-friendly, and safer medical treatments and supplies, highlighting the market's potential for continued expansion and innovation in the coming years.
| Report Metric | Details |
| Report Name | Zinc Oxide Adhesive Plaster Market |
| Accounted market size in 2023 | US$ 1536 million |
| Forecasted market size in 2030 | US$ 2571.9 million |
| CAGR | 7.7% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Smith and Nephew, Johnson and Johnson, 3M, Beiersdorf AG, Winner Medical Group, McKesson Corporation, B. Braun, Paul Hartmann AG, Sterimed, Henso Medical, BOENMED, Unimax Medical, Forlong Medical, AdvaCare Pharma, Changzhou Medical Appliances General Factory, Proexamine Surgicals, Greetmed |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |