What is Global Varicella Attenuated Live Vaccination Market?
The Global Varicella Attenuated Live Vaccination Market refers to the worldwide industry focused on the development, production, and distribution of vaccines designed to prevent varicella, commonly known as chickenpox. This market is centered around vaccines that use a live, but weakened, form of the varicella-zoster virus to stimulate an immune response without causing the disease itself. These vaccines are crucial in reducing the incidence of chickenpox, which is a highly contagious disease characterized by an itchy rash and flu-like symptoms. The market encompasses various stakeholders, including pharmaceutical companies, healthcare providers, and regulatory bodies, all working together to ensure the availability and accessibility of these vaccines. The demand for varicella vaccines is driven by factors such as increasing awareness of vaccination benefits, government immunization programs, and the need to prevent outbreaks in communities. As the global population continues to grow and healthcare infrastructure improves, the market for varicella attenuated live vaccines is expected to expand, providing significant opportunities for innovation and development in vaccine technology. This market plays a vital role in public health by contributing to the control and eventual eradication of chickenpox worldwide.
Monovalent Vaccine, Combination Vaccine in the Global Varicella Attenuated Live Vaccination Market:
In the Global Varicella Attenuated Live Vaccination Market, vaccines are primarily categorized into two types: monovalent vaccines and combination vaccines. Monovalent vaccines are designed to immunize against a single antigen or microorganism, in this case, the varicella-zoster virus. These vaccines are specifically formulated to prevent chickenpox and are typically administered to children as part of routine immunization schedules. Monovalent varicella vaccines have been instrumental in significantly reducing the incidence of chickenpox, leading to fewer hospitalizations and complications associated with the disease. They are often recommended for individuals who have not been previously vaccinated or have not had chickenpox, providing a targeted approach to disease prevention. On the other hand, combination vaccines contain multiple antigens, allowing them to protect against more than one disease with a single injection. In the context of varicella vaccination, combination vaccines may include protection against diseases such as measles, mumps, and rubella, in addition to chickenpox. These vaccines are particularly advantageous in pediatric immunization programs, as they reduce the number of injections required, thereby improving compliance and coverage rates. Combination vaccines are also beneficial in resource-limited settings where healthcare access may be restricted, as they simplify logistics and reduce the burden on healthcare systems. The development and distribution of both monovalent and combination varicella vaccines are influenced by various factors, including regulatory approvals, manufacturing capabilities, and market demand. Pharmaceutical companies invest heavily in research and development to enhance the efficacy, safety, and stability of these vaccines, ensuring they meet stringent quality standards. Additionally, collaborations between governments, international organizations, and private sector entities play a crucial role in expanding vaccine access and addressing global health challenges. As the Global Varicella Attenuated Live Vaccination Market continues to evolve, the focus remains on improving vaccine formulations, delivery methods, and distribution networks to maximize public health benefits. The ongoing efforts to enhance vaccine coverage and address vaccine hesitancy are essential in achieving the ultimate goal of controlling and potentially eradicating chickenpox worldwide.
Children, Adults in the Global Varicella Attenuated Live Vaccination Market:
The usage of the Global Varicella Attenuated Live Vaccination Market extends to various demographic groups, with specific considerations for children and adults. In children, varicella vaccination is a critical component of routine immunization schedules, typically administered in two doses to ensure optimal protection. The first dose is usually given between 12 and 15 months of age, followed by a second dose between 4 and 6 years of age. This vaccination strategy has proven highly effective in reducing the incidence of chickenpox among children, leading to a significant decline in related complications, such as bacterial infections, pneumonia, and encephalitis. By preventing chickenpox in children, the vaccine also contributes to herd immunity, reducing the overall transmission of the virus within communities. In addition to routine childhood vaccination, the varicella vaccine is recommended for certain groups of adults who are at increased risk of exposure or complications from chickenpox. This includes healthcare workers, teachers, and individuals who have not previously been vaccinated or had the disease. For adults, the vaccination schedule may vary based on individual health status and risk factors, with some requiring a single dose and others needing two doses for full protection. The vaccine is particularly important for adults who are immunocompromised or have chronic health conditions, as they are more susceptible to severe outcomes from chickenpox. Furthermore, the varicella vaccine plays a crucial role in preventing shingles, a painful condition caused by the reactivation of the varicella-zoster virus in individuals who have previously had chickenpox. While the primary focus of the Global Varicella Attenuated Live Vaccination Market is on preventing chickenpox, the broader implications for public health are significant. By reducing the incidence of chickenpox and its complications, the vaccine alleviates the burden on healthcare systems, decreases healthcare costs, and improves quality of life for individuals and communities. The ongoing efforts to increase vaccine coverage and address barriers to access are essential in maximizing the benefits of varicella vaccination for both children and adults.
Global Varicella Attenuated Live Vaccination Market Outlook:
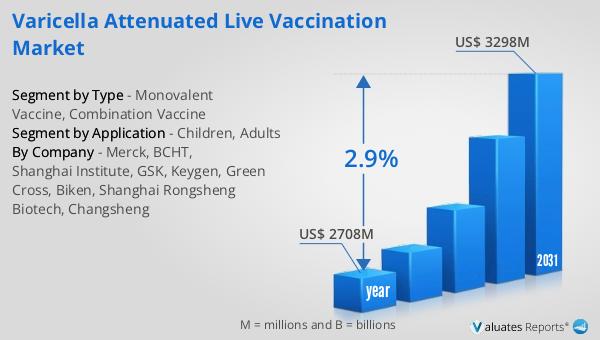
The global market for Varicella Attenuated Live Vaccination was valued at $2,708 million in 2024 and is anticipated to grow to a revised size of $3,298 million by 2031, reflecting a compound annual growth rate (CAGR) of 2.9% over the forecast period. This growth trajectory underscores the increasing demand and importance of varicella vaccines in global healthcare. In comparison, the broader pharmaceutical market was valued at $1,475 billion in 2022, with an expected CAGR of 5% over the next six years, indicating robust growth across the pharmaceutical sector. Meanwhile, the chemical drug market, a significant segment within the pharmaceutical industry, was estimated to grow from $1,005 billion in 2018 to $1,094 billion in 2022. These figures highlight the dynamic nature of the pharmaceutical landscape, with the varicella vaccine market playing a crucial role in addressing public health needs. The steady growth of the Varicella Attenuated Live Vaccination Market reflects ongoing efforts to enhance vaccine accessibility, improve immunization rates, and address challenges such as vaccine hesitancy and logistical barriers. As healthcare systems worldwide continue to prioritize vaccination programs, the market for varicella vaccines is poised to make significant contributions to global health outcomes.
| Report Metric | Details |
| Report Name | Varicella Attenuated Live Vaccination Market |
| Accounted market size in year | US$ 2708 million |
| Forecasted market size in 2031 | US$ 3298 million |
| CAGR | 2.9% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Merck, BCHT, Shanghai Institute, GSK, Keygen, Green Cross, Biken, Shanghai Rongsheng Biotech, Changsheng |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |