What is Global API Process Development and Manufacturing Market?
The Global API Process Development and Manufacturing Market refers to the worldwide industry focused on the creation and production of Active Pharmaceutical Ingredients (APIs). APIs are the essential components in drugs that produce the intended effects. This market encompasses a wide range of activities, from the initial development of the API to its large-scale manufacturing. The process involves rigorous research, development, and testing to ensure that the APIs meet stringent quality and safety standards. The market is driven by the increasing demand for pharmaceuticals, advancements in technology, and the need for cost-effective production methods. Companies in this market work on developing both small molecule APIs, which are chemically synthesized, and large molecule APIs, which are typically produced using biological processes. The market is highly competitive, with numerous players striving to innovate and improve their processes to gain a competitive edge. The ultimate goal is to produce high-quality APIs that can be used in the formulation of effective and safe medications for various health conditions.
Small Molecule API Process Development and Manufacturing, Large Molecule API Process Development and Manufacturing in the Global API Process Development and Manufacturing Market:
Small Molecule API Process Development and Manufacturing and Large Molecule API Process Development and Manufacturing are two critical segments within the Global API Process Development and Manufacturing Market. Small molecule APIs are typically low molecular weight compounds that are chemically synthesized. These APIs are used in a wide range of pharmaceutical products, including tablets, capsules, and injectables. The development process for small molecule APIs involves several stages, including discovery, preclinical testing, clinical trials, and regulatory approval. Each stage requires meticulous planning and execution to ensure that the API is both effective and safe for use. On the other hand, large molecule APIs, also known as biologics, are typically high molecular weight compounds produced using biological processes. These APIs include proteins, peptides, and monoclonal antibodies, which are used in the treatment of various diseases, including cancer, autoimmune disorders, and infectious diseases. The development process for large molecule APIs is more complex and time-consuming compared to small molecule APIs. It involves the use of living cells or organisms to produce the desired compound, followed by purification and characterization to ensure its quality and efficacy. Both small and large molecule API development require significant investment in research and development, as well as advanced manufacturing technologies to produce high-quality products. The choice between small and large molecule APIs depends on various factors, including the nature of the disease being treated, the route of administration, and the desired therapeutic effect. Companies in the Global API Process Development and Manufacturing Market must continuously innovate and improve their processes to meet the evolving needs of the pharmaceutical industry and ensure the availability of safe and effective medications for patients worldwide.
Pharmaceutical, Clinical, Others in the Global API Process Development and Manufacturing Market:
The Global API Process Development and Manufacturing Market plays a crucial role in various areas, including pharmaceutical, clinical, and other sectors. In the pharmaceutical sector, APIs are the backbone of drug development and production. Pharmaceutical companies rely on high-quality APIs to formulate effective medications for a wide range of health conditions. The process of developing and manufacturing APIs involves rigorous testing and quality control to ensure that the final product meets regulatory standards and is safe for patient use. In the clinical sector, APIs are essential for conducting clinical trials and research studies. Clinical trials are a critical step in the drug development process, as they help determine the safety and efficacy of new medications. APIs used in clinical trials must be produced under strict conditions to ensure their purity and consistency. Additionally, the Global API Process Development and Manufacturing Market supports other sectors, such as biotechnology and contract manufacturing organizations (CMOs). Biotechnology companies often rely on APIs for the development of innovative therapies, including gene and cell therapies. CMOs provide specialized services in API development and manufacturing, helping pharmaceutical companies bring their products to market more efficiently. The market also supports the production of generic drugs, which are essential for providing affordable medications to patients. Overall, the Global API Process Development and Manufacturing Market is integral to the healthcare industry, ensuring the availability of high-quality APIs for the development of safe and effective medications.
Global API Process Development and Manufacturing Market Outlook:
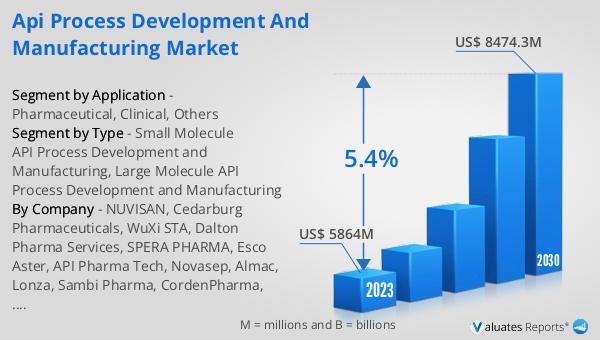
The global API Process Development and Manufacturing market was valued at US$ 5864 million in 2023 and is anticipated to reach US$ 8474.3 million by 2030, witnessing a CAGR of 5.4% during the forecast period 2024-2030. This market outlook highlights the significant growth potential of the industry, driven by increasing demand for pharmaceuticals and advancements in technology. The market's value in 2023 underscores its importance in the healthcare sector, providing essential components for drug development and production. The projected growth to US$ 8474.3 million by 2030 indicates a robust expansion, reflecting the continuous need for high-quality APIs. The compound annual growth rate (CAGR) of 5.4% during the forecast period further emphasizes the market's dynamic nature and the opportunities for innovation and development. Companies operating in this market must focus on enhancing their research and development capabilities, adopting advanced manufacturing technologies, and ensuring compliance with regulatory standards to capitalize on this growth. The market outlook serves as a valuable indicator for stakeholders, including pharmaceutical companies, investors, and policymakers, highlighting the critical role of the Global API Process Development and Manufacturing Market in the future of healthcare.
| Report Metric | Details |
| Report Name | API Process Development and Manufacturing Market |
| Accounted market size in 2023 | US$ 5864 million |
| Forecasted market size in 2030 | US$ 8474.3 million |
| CAGR | 5.4% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | NUVISAN, Cedarburg Pharmaceuticals, WuXi STA, Dalton Pharma Services, SPERA PHARMA, Esco Aster, API Pharma Tech, Novasep, Almac, Lonza, Sambi Pharma, CordenPharma, Carbogen Amcis, Azis Labs, Biocon |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
