What is Global Charcot-Marie-Tooth Disease Type I A Drug Market?
The Global Charcot-Marie-Tooth Disease Type I A Drug Market is a specialized segment within the broader pharmaceutical industry, focusing on treatments for a rare genetic disorder known as Charcot-Marie-Tooth (CMT) disease. CMT is a hereditary neuropathy that affects the peripheral nerves, leading to muscle weakness and atrophy, primarily in the legs and feet, and sometimes in the hands. Type I A is the most common form of CMT, caused by a duplication of the PMP22 gene on chromosome 17. The market for drugs targeting this condition is driven by the need for effective therapies that can alleviate symptoms, slow disease progression, and improve the quality of life for patients. Currently, there is no cure for CMT, and treatment options are limited, which underscores the importance of ongoing research and development in this field. Pharmaceutical companies are investing in innovative drug candidates and exploring various therapeutic approaches, including gene therapy, to address the unmet medical needs of CMT patients. The market is characterized by a growing pipeline of potential treatments, collaborations between biotech firms and research institutions, and an increasing awareness of the disease among healthcare professionals and patients. As the understanding of CMT Type I A advances, the development of targeted therapies is expected to expand, offering hope to those affected by this challenging condition.
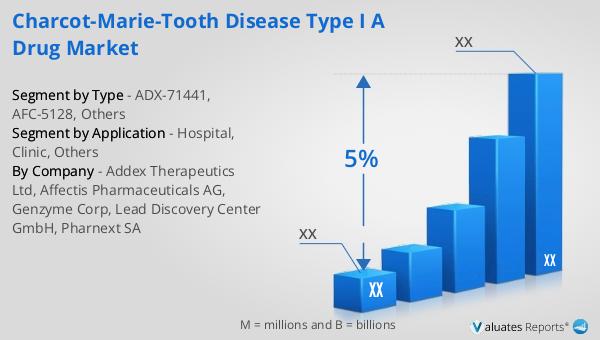
ADX-71441, AFC-5128, Others in the Global Charcot-Marie-Tooth Disease Type I A Drug Market:
In the Global Charcot-Marie-Tooth Disease Type I A Drug Market, several promising drug candidates are under investigation, including ADX-71441, AFC-5128, and others. ADX-71441 is a novel compound that acts as a positive allosteric modulator of the GABA-B receptor. This mechanism of action is believed to enhance the inhibitory effects of GABA, a neurotransmitter that plays a crucial role in reducing neuronal excitability. By modulating the GABA-B receptor, ADX-71441 aims to alleviate neuropathic pain and improve motor function in patients with CMT Type I A. Preclinical studies have shown promising results, and the drug is currently undergoing clinical trials to evaluate its safety and efficacy in humans. AFC-5128, on the other hand, is a small molecule drug that targets the underlying genetic cause of CMT Type I A. It is designed to reduce the overexpression of the PMP22 gene, which is responsible for the demyelination of peripheral nerves in affected individuals. By normalizing PMP22 levels, AFC-5128 has the potential to restore nerve function and halt disease progression. This approach represents a significant advancement in the treatment of CMT, as it addresses the root cause of the disorder rather than just managing symptoms. Other drug candidates in the pipeline include gene therapies and antisense oligonucleotides, which aim to correct the genetic mutations responsible for CMT Type I A. These innovative therapies are being developed in collaboration with leading research institutions and biotech companies, leveraging cutting-edge technologies to deliver targeted treatments. The development of these drugs is supported by a growing body of scientific research and clinical data, which highlights the potential of these therapies to transform the treatment landscape for CMT patients. As these drug candidates progress through clinical trials, they offer hope for improved outcomes and a better quality of life for individuals living with Charcot-Marie-Tooth Disease Type I A. The market for these drugs is expected to expand as more therapies receive regulatory approval and become available to patients worldwide. This expansion is driven by the increasing prevalence of CMT, advancements in genetic research, and a greater understanding of the disease's pathophysiology. The collaboration between pharmaceutical companies, academic institutions, and patient advocacy groups is also playing a crucial role in accelerating the development of new treatments and raising awareness about CMT. As the pipeline of potential therapies continues to grow, the Global Charcot-Marie-Tooth Disease Type I A Drug Market is poised to make significant strides in addressing the unmet needs of patients and improving their quality of life.
Hospital, Clinic, Others in the Global Charcot-Marie-Tooth Disease Type I A Drug Market:
The usage of drugs in the Global Charcot-Marie-Tooth Disease Type I A Drug Market spans various healthcare settings, including hospitals, clinics, and other specialized care facilities. In hospitals, these drugs are often administered as part of a comprehensive treatment plan for patients with severe symptoms or complications related to CMT Type I A. Hospitals provide a multidisciplinary approach to care, involving neurologists, physiotherapists, and other specialists who work together to manage the disease and its associated symptoms. The availability of advanced diagnostic tools and specialized medical staff in hospitals ensures that patients receive accurate diagnoses and personalized treatment plans. In clinics, the focus is often on outpatient care and ongoing management of CMT Type I A. Clinics provide a more accessible and convenient setting for patients to receive regular check-ups, medication adjustments, and supportive therapies. Healthcare providers in clinics work closely with patients to monitor their progress, address any side effects of medications, and provide guidance on lifestyle modifications that can help manage the disease. Clinics also play a vital role in educating patients and their families about CMT, empowering them to take an active role in their care. Other settings where CMT Type I A drugs are used include rehabilitation centers and home healthcare services. Rehabilitation centers offer specialized programs designed to improve mobility, strength, and overall function in patients with CMT. These programs often incorporate physical therapy, occupational therapy, and assistive devices to help patients maintain independence and enhance their quality of life. Home healthcare services provide an alternative for patients who may have difficulty accessing traditional healthcare facilities due to mobility issues or other constraints. These services offer personalized care in the comfort of the patient's home, ensuring that they receive the necessary medications and therapies to manage their condition effectively. The integration of CMT Type I A drugs into these various healthcare settings highlights the importance of a coordinated and patient-centered approach to care. By addressing the unique needs of each patient and providing tailored treatment plans, healthcare providers can optimize outcomes and improve the overall quality of life for individuals living with Charcot-Marie-Tooth Disease Type I A. As new therapies become available, the role of these healthcare settings will continue to evolve, offering patients more options and greater access to innovative treatments.
Global Charcot-Marie-Tooth Disease Type I A Drug Market Outlook:
The outlook for the Global Charcot-Marie-Tooth Disease Type I A Drug Market can be contextualized within the broader pharmaceutical industry trends. In 2022, the global pharmaceutical market was valued at approximately 1,475 billion USD, with an anticipated compound annual growth rate (CAGR) of 5% over the next six years. This growth is indicative of the increasing demand for innovative therapies and the expansion of healthcare access worldwide. In comparison, the chemical drug market, a significant subset of the pharmaceutical industry, was projected to grow from 1,005 billion USD in 2018 to 1,094 billion USD by 2022. This growth reflects the ongoing advancements in drug development and the introduction of new chemical entities that address various medical conditions, including rare diseases like Charcot-Marie-Tooth Disease Type I A. The market dynamics for CMT Type I A drugs are influenced by these broader industry trends, as well as the specific challenges and opportunities associated with developing treatments for rare genetic disorders. The increasing focus on personalized medicine, advancements in genetic research, and the growing collaboration between pharmaceutical companies and research institutions are key drivers of innovation in this market. As the understanding of CMT Type I A continues to evolve, the development of targeted therapies is expected to accelerate, offering new hope to patients and their families. The market outlook for CMT Type I A drugs is promising, with the potential for significant advancements in treatment options and improved patient outcomes.
| Report Metric | Details |
| Report Name | Charcot-Marie-Tooth Disease Type I A Drug Market |
| CAGR | 5% |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Addex Therapeutics Ltd, Affectis Pharmaceuticals AG, Genzyme Corp, Lead Discovery Center GmbH, Pharnext SA |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |