What is Global Burkholderia Pseudomallei Infections Drug Market?
The Global Burkholderia Pseudomallei Infections Drug Market is a specialized segment within the broader pharmaceutical industry, focusing on treatments for infections caused by the Burkholderia pseudomallei bacterium. This bacterium is responsible for melioidosis, a disease prevalent in tropical regions, particularly in Southeast Asia and Northern Australia. The market for drugs targeting this infection is driven by the need for effective treatments due to the bacterium's resistance to many common antibiotics. The market encompasses a range of pharmaceutical products, including antibiotics specifically designed to combat this resilient pathogen. Research and development in this field are crucial, as the disease can be fatal if not treated properly. The market's growth is influenced by factors such as increased awareness of the disease, advancements in medical research, and the development of new and more effective drugs. As global travel and climate change potentially expand the regions affected by melioidosis, the demand for effective treatments is expected to rise, making this a critical area of focus for pharmaceutical companies and healthcare providers worldwide.
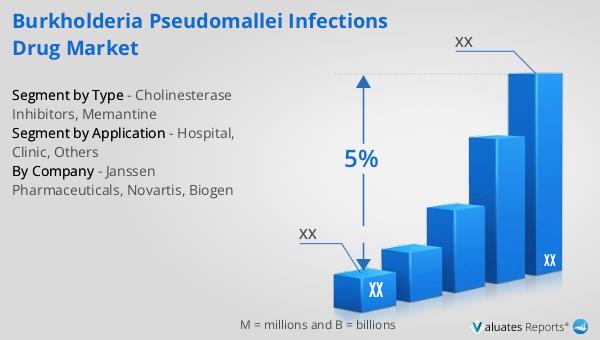
Cholinesterase Inhibitors, Memantine in the Global Burkholderia Pseudomallei Infections Drug Market:
Cholinesterase inhibitors and Memantine are not directly related to the Global Burkholderia Pseudomallei Infections Drug Market, as they are primarily used in the treatment of Alzheimer's disease and other forms of dementia. However, understanding their role in the pharmaceutical landscape can provide insights into how different drug markets operate. Cholinesterase inhibitors, such as Donepezil, Rivastigmine, and Galantamine, work by preventing the breakdown of acetylcholine, a neurotransmitter important for memory and learning. By increasing the levels of acetylcholine in the brain, these drugs can help improve cognitive function and slow the progression of symptoms in patients with Alzheimer's disease. Memantine, on the other hand, works differently. It is an NMDA receptor antagonist that helps regulate the activity of glutamate, another neurotransmitter involved in learning and memory. By modulating glutamate activity, Memantine can help protect brain cells from damage and improve cognitive function in patients with moderate to severe Alzheimer's disease. While these drugs are not used to treat Burkholderia pseudomallei infections, their development and use highlight the importance of targeted therapies in addressing specific medical conditions. The pharmaceutical industry invests heavily in research and development to create drugs that can effectively target the underlying causes of diseases, whether they are neurological disorders like Alzheimer's or bacterial infections like melioidosis. The success of Cholinesterase inhibitors and Memantine in the market demonstrates the potential for targeted therapies to improve patient outcomes and enhance quality of life. In the context of the Global Burkholderia Pseudomallei Infections Drug Market, similar principles apply. The development of effective antibiotics and treatments for melioidosis requires a deep understanding of the bacterium's biology and the mechanisms by which it causes disease. Pharmaceutical companies and researchers must work together to identify new drug targets and develop therapies that can overcome the bacterium's resistance to existing antibiotics. This process involves extensive research, clinical trials, and collaboration between scientists, healthcare providers, and regulatory agencies. The goal is to create safe and effective treatments that can be used to manage and ultimately eradicate Burkholderia pseudomallei infections. As with Cholinesterase inhibitors and Memantine, the success of these efforts depends on a combination of scientific innovation, regulatory support, and market demand. The Global Burkholderia Pseudomallei Infections Drug Market is a testament to the ongoing need for targeted therapies in the fight against infectious diseases. By investing in research and development, pharmaceutical companies can help ensure that patients have access to the treatments they need to combat this challenging and potentially deadly infection.
Hospital, Clinic, Others in the Global Burkholderia Pseudomallei Infections Drug Market:
The usage of drugs from the Global Burkholderia Pseudomallei Infections Drug Market is crucial in various healthcare settings, including hospitals, clinics, and other medical facilities. In hospitals, these drugs are often used in the treatment of severe cases of melioidosis, where patients require intensive care and monitoring. Hospitals are equipped with the necessary resources and expertise to manage complex cases, making them a primary setting for the administration of these specialized antibiotics. In such environments, healthcare professionals can closely monitor patients' responses to treatment, adjust dosages as needed, and manage any potential side effects. This level of care is essential for patients with severe infections, as timely and effective treatment can significantly improve outcomes and reduce the risk of complications. In clinics, the use of Burkholderia pseudomallei infection drugs is typically focused on the diagnosis and management of less severe cases. Clinics serve as an important point of access for patients who may not require hospitalization but still need medical intervention. In these settings, healthcare providers can prescribe appropriate antibiotics and provide guidance on managing the infection at home. Clinics also play a vital role in early detection and prevention, as they are often the first point of contact for patients experiencing symptoms of melioidosis. By identifying and treating the infection early, clinics can help prevent the progression of the disease and reduce the burden on hospital resources. Other healthcare settings, such as community health centers and specialized infectious disease units, also play a role in the management of Burkholderia pseudomallei infections. These facilities may focus on providing education and support to patients and their families, as well as conducting research to improve treatment protocols and outcomes. In addition, public health initiatives aimed at raising awareness of melioidosis and promoting preventive measures are essential in reducing the incidence of the disease. These efforts may include community outreach programs, educational campaigns, and collaboration with local and international health organizations. Overall, the Global Burkholderia Pseudomallei Infections Drug Market plays a critical role in supporting healthcare providers across various settings in their efforts to diagnose, treat, and prevent this challenging infection. By ensuring access to effective treatments and promoting awareness of the disease, the market helps improve patient outcomes and contributes to the broader goal of reducing the global impact of melioidosis.
Global Burkholderia Pseudomallei Infections Drug Market Outlook:
In 2022, the global pharmaceutical market reached a valuation of 1,475 billion USD, marking a significant milestone in the industry. This market is projected to grow at a compound annual growth rate (CAGR) of 5% over the next six years, reflecting the ongoing demand for innovative medical treatments and therapies. In comparison, the chemical drug market has also shown substantial growth. From 2018 to 2022, the market expanded from 1,005 billion USD to 1,094 billion USD. This growth underscores the importance of chemical drugs in the pharmaceutical landscape, as they continue to play a vital role in treating a wide range of medical conditions. The increase in market size for both the overall pharmaceutical industry and the chemical drug segment highlights the dynamic nature of the healthcare sector. Factors such as advancements in medical research, the development of new drug formulations, and the rising prevalence of chronic diseases contribute to this growth. Additionally, the expansion of healthcare infrastructure in emerging markets and increased access to medical care are driving demand for pharmaceutical products worldwide. As the industry continues to evolve, companies are investing in research and development to create innovative solutions that address unmet medical needs. This focus on innovation is essential for maintaining the momentum of growth in the pharmaceutical market and ensuring that patients have access to effective and safe treatments. The Global Burkholderia Pseudomallei Infections Drug Market is a part of this broader landscape, contributing to the development of targeted therapies for specific infectious diseases. By understanding the trends and dynamics of the pharmaceutical market, stakeholders can better navigate the challenges and opportunities that lie ahead.
| Report Metric | Details |
| Report Name | Burkholderia Pseudomallei Infections Drug Market |
| CAGR | 5% |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Janssen Pharmaceuticals, Novartis, Biogen |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
![Superoxide Dismutase [Cu-Zn] Market](https://ilu.valuates.com/5248535786160128/superoxide-dismutase--cu-zn--market-600w.jpg)