What is Global Hypereosinophilic Syndrome Drug Market?
The Global Hypereosinophilic Syndrome Drug Market is a specialized segment within the broader pharmaceutical industry, focusing on treatments for hypereosinophilic syndrome (HES), a rare and chronic condition characterized by an overproduction of eosinophils, a type of white blood cell. This overproduction can lead to inflammation and damage in various organs, including the heart, lungs, skin, and nervous system. The market for HES drugs is driven by the need for effective therapies that can manage symptoms and improve the quality of life for patients. With advancements in medical research and biotechnology, several drugs have been developed and are in various stages of clinical trials or have already been approved for use. These drugs aim to target the underlying causes of HES, reduce eosinophil levels, and alleviate the associated symptoms. The market is characterized by ongoing research and development efforts, collaborations between pharmaceutical companies, and a growing awareness of the condition among healthcare professionals. As a result, the Global Hypereosinophilic Syndrome Drug Market is poised for growth, with an increasing number of treatment options becoming available to patients worldwide.
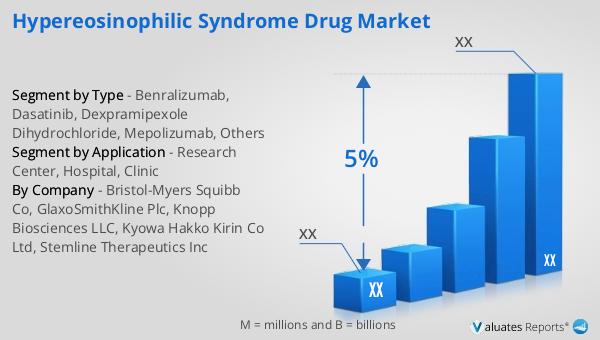
Benralizumab, Dasatinib, Dexpramipexole Dihydrochloride, Mepolizumab, Others in the Global Hypereosinophilic Syndrome Drug Market:
Benralizumab is one of the key drugs in the Global Hypereosinophilic Syndrome Drug Market. It is a monoclonal antibody that targets the interleukin-5 receptor on eosinophils, leading to their depletion. This drug is particularly effective in reducing eosinophil levels and controlling symptoms in patients with severe eosinophilic conditions. Clinical trials have shown that Benralizumab can significantly improve lung function and reduce the frequency of exacerbations in patients with eosinophilic asthma, which shares some pathophysiological features with HES. Dasatinib, originally developed as a treatment for chronic myeloid leukemia, has shown promise in treating HES due to its ability to inhibit certain tyrosine kinases involved in eosinophil proliferation. It is particularly useful in cases where HES is associated with specific genetic mutations. Dexpramipexole Dihydrochloride is another drug under investigation for its potential to reduce eosinophil levels. It works by modulating the immune response and has shown efficacy in early clinical trials. Mepolizumab, like Benralizumab, is an anti-IL-5 monoclonal antibody that has been approved for use in eosinophilic asthma and is being explored for its effectiveness in HES. It works by directly targeting and neutralizing IL-5, a cytokine crucial for eosinophil growth and survival. Other drugs in the market include those targeting different pathways involved in eosinophil activation and survival, offering a range of options for personalized treatment approaches. The development of these drugs is supported by extensive research and clinical trials, which are essential for understanding their safety, efficacy, and optimal use in managing HES. As the market evolves, there is a continuous effort to identify new therapeutic targets and develop innovative treatments that can provide better outcomes for patients with this challenging condition.
Research Center, Hospital, Clinic in the Global Hypereosinophilic Syndrome Drug Market:
The usage of drugs from the Global Hypereosinophilic Syndrome Drug Market extends across various healthcare settings, including research centers, hospitals, and clinics. In research centers, these drugs are at the forefront of scientific investigation, with researchers exploring their mechanisms of action, potential side effects, and efficacy in different patient populations. Clinical trials conducted in these centers are crucial for gaining regulatory approval and ensuring that the drugs are safe and effective for widespread use. Hospitals play a vital role in the administration and monitoring of these drugs, particularly for patients with severe or complicated cases of HES. In a hospital setting, patients can receive comprehensive care, including diagnostic evaluations, drug administration, and management of any adverse reactions. Hospitals also serve as centers for patient education, where healthcare professionals can provide information on the condition and the available treatment options. Clinics, on the other hand, offer a more accessible and convenient setting for ongoing management of HES. In clinics, patients can receive regular follow-ups, medication adjustments, and supportive care to manage their symptoms and improve their quality of life. The availability of HES drugs in these settings ensures that patients have access to the latest treatments and can benefit from a multidisciplinary approach to their care. This integrated approach is essential for optimizing treatment outcomes and ensuring that patients receive the best possible care for their condition.
Global Hypereosinophilic Syndrome Drug Market Outlook:
The outlook for the Global Hypereosinophilic Syndrome Drug Market is closely tied to the broader trends in the pharmaceutical industry. In 2022, the global pharmaceutical market was valued at approximately 1,475 billion USD, with a projected compound annual growth rate (CAGR) of 5% over the next six years. This growth is indicative of the increasing demand for innovative therapies and the expansion of healthcare access worldwide. In comparison, the chemical drug market, a subset of the pharmaceutical industry, was estimated to grow from 1,005 billion USD in 2018 to 1,094 billion USD in 2022. This growth reflects the ongoing development and commercialization of new chemical entities, including those targeting rare diseases like hypereosinophilic syndrome. The market dynamics for HES drugs are influenced by factors such as advancements in biotechnology, increased awareness of rare diseases, and the need for personalized medicine approaches. As the pharmaceutical industry continues to evolve, the Global Hypereosinophilic Syndrome Drug Market is expected to benefit from these trends, with more treatment options becoming available to patients and healthcare providers. This growth is essential for addressing the unmet medical needs of patients with HES and improving their overall health outcomes.
| Report Metric | Details |
| Report Name | Hypereosinophilic Syndrome Drug Market |
| CAGR | 5% |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Bristol-Myers Squibb Co, GlaxoSmithKline Plc, Knopp Biosciences LLC, Kyowa Hakko Kirin Co Ltd, Stemline Therapeutics Inc |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |