What is Global Angiography Surgery Vascular Closure Device (VCD) Market?
The Global Angiography Surgery Vascular Closure Device (VCD) Market is a specialized segment within the broader medical device industry, focusing on devices used to close vascular access sites post-angiography procedures. Angiography is a medical imaging technique used to visualize the inside of blood vessels and organs, particularly to identify blockages or abnormalities. After such procedures, it's crucial to close the vascular access site efficiently to prevent complications such as bleeding or infection. Vascular closure devices (VCDs) are designed to achieve hemostasis, or the stopping of blood flow, quickly and effectively. These devices are particularly important in procedures involving the femoral artery, where traditional manual compression can be time-consuming and uncomfortable for patients. The market for these devices is driven by the increasing number of angiography procedures worldwide, advancements in technology, and a growing preference for minimally invasive procedures. As healthcare systems globally strive for efficiency and improved patient outcomes, the demand for effective VCDs continues to rise, making this market a critical component of modern medical practices.
5F, 6F, 7F, 8F, Others in the Global Angiography Surgery Vascular Closure Device (VCD) Market:
In the Global Angiography Surgery Vascular Closure Device (VCD) Market, the terms 5F, 6F, 7F, 8F, and others refer to the French gauge size of the catheters used during angiography procedures. The French gauge system is a measure of the outer diameter of a catheter, with one French unit equating to one-third of a millimeter. The choice of catheter size is crucial as it directly impacts the procedure's success and the patient's comfort. A 5F catheter, for instance, has a smaller diameter and is often used in procedures requiring less invasive access, making it suitable for patients with smaller vessels or when the procedure's complexity is minimal. On the other hand, a 6F catheter is more commonly used in standard angiography procedures, providing a balance between ease of access and the ability to accommodate various diagnostic and therapeutic tools. The 7F and 8F catheters are typically reserved for more complex procedures or when larger devices need to be introduced into the vascular system. These larger sizes allow for greater flexibility and the ability to perform more intricate interventions. The choice of catheter size also influences the type of vascular closure device used post-procedure. Smaller catheters may require different closure techniques compared to larger ones, as the size of the access site directly affects the closure method's effectiveness. For instance, a smaller access site may be closed using a simple suture-based device, while larger sites might necessitate more advanced closure systems that use collagen plugs or other materials to achieve hemostasis. The market for these devices is diverse, with manufacturers offering a range of products tailored to different catheter sizes and procedural requirements. This diversity ensures that healthcare providers can select the most appropriate device for each patient's unique needs, enhancing the overall success of the procedure and minimizing complications. As the field of angiography continues to evolve, so too does the technology behind vascular closure devices. Innovations in materials, design, and functionality are continually being developed to improve patient outcomes and streamline procedural efficiency. This ongoing advancement is a testament to the critical role that catheter size and corresponding closure devices play in the angiography landscape. The ability to choose the right size and type of device is not only a matter of technical precision but also a key factor in ensuring patient safety and satisfaction. As such, the Global Angiography Surgery Vascular Closure Device (VCD) Market remains a dynamic and essential component of the medical device industry, continually adapting to meet the needs of healthcare providers and patients alike.
Hospital, Clinic in the Global Angiography Surgery Vascular Closure Device (VCD) Market:
The usage of Global Angiography Surgery Vascular Closure Devices (VCDs) in hospitals and clinics is integral to the efficiency and safety of angiographic procedures. In hospitals, where the volume of angiography procedures is typically high, VCDs play a crucial role in streamlining operations and improving patient throughput. Hospitals often deal with a wide range of cases, from routine diagnostic angiographies to complex interventional procedures. In such settings, the ability to quickly and effectively close vascular access sites is paramount. VCDs reduce the time required for hemostasis compared to manual compression, allowing patients to mobilize sooner and freeing up valuable hospital resources. This efficiency is particularly beneficial in busy catheterization labs, where time and space are at a premium. Moreover, the use of VCDs in hospitals contributes to improved patient outcomes by minimizing the risk of complications such as bleeding or infection, which can lead to extended hospital stays and increased healthcare costs. In clinics, where the scale of operations might be smaller, the use of VCDs is equally important. Clinics often cater to outpatient procedures, where patient turnover is critical. The ability to achieve rapid hemostasis with VCDs means that patients can be discharged sooner, enhancing the clinic's capacity to handle more cases. This is particularly advantageous in settings where resources are limited, and efficiency is key to maintaining a viable operation. Additionally, the use of VCDs in clinics can enhance patient satisfaction by reducing the discomfort associated with prolonged bed rest and manual compression. Patients appreciate the quicker recovery times and the ability to return to their daily activities sooner, which is a significant consideration in outpatient care. Both hospitals and clinics benefit from the technological advancements in VCDs, which have led to devices that are easier to use, more reliable, and tailored to specific procedural needs. The availability of a range of VCDs designed for different catheter sizes and patient anatomies ensures that healthcare providers can choose the most appropriate device for each situation. This adaptability is crucial in delivering personalized care and optimizing procedural outcomes. As the demand for angiographic procedures continues to grow, driven by an aging population and the increasing prevalence of cardiovascular diseases, the role of VCDs in hospitals and clinics becomes even more significant. These devices not only enhance procedural efficiency and patient safety but also contribute to the overall sustainability of healthcare systems by reducing costs and improving resource utilization. In summary, the use of Global Angiography Surgery Vascular Closure Devices in hospitals and clinics is a vital component of modern healthcare, supporting the delivery of high-quality, efficient, and patient-centered care.
Global Angiography Surgery Vascular Closure Device (VCD) Market Outlook:
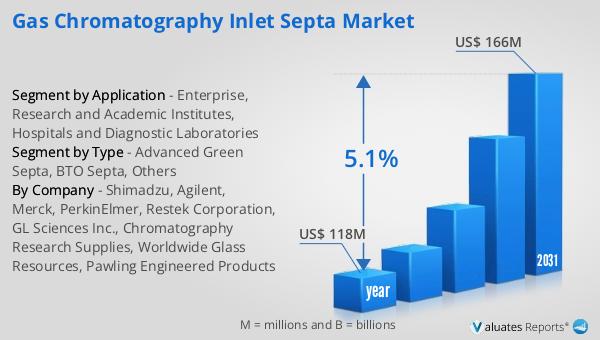
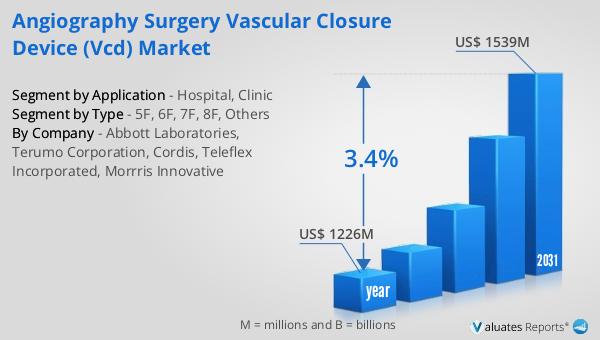
The global market for Angiography Surgery Vascular Closure Devices (VCDs) was valued at approximately $1,226 million in 2024, with projections indicating it will grow to around $1,539 million by 2031. This growth represents a compound annual growth rate (CAGR) of 3.4% over the forecast period. This market expansion is reflective of the broader trends within the medical device industry, which was estimated to be worth $603 billion in 2023 and is expected to grow at a CAGR of 5% over the next six years. The steady growth in the VCD market can be attributed to several factors, including the increasing number of angiography procedures, advancements in device technology, and a growing emphasis on minimally invasive surgical techniques. As healthcare systems worldwide continue to prioritize patient safety and procedural efficiency, the demand for reliable and effective vascular closure solutions is expected to rise. The market's growth is also supported by the ongoing development of new materials and designs that enhance the performance and ease of use of VCDs. These innovations are crucial in meeting the diverse needs of healthcare providers and patients, ensuring that the market remains dynamic and responsive to changing demands. In conclusion, the Global Angiography Surgery Vascular Closure Device Market is poised for steady growth, driven by technological advancements and an increasing focus on improving patient outcomes.
| Report Metric | Details |
| Report Name | Angiography Surgery Vascular Closure Device (VCD) Market |
| Accounted market size in year | US$ 1226 million |
| Forecasted market size in 2031 | US$ 1539 million |
| CAGR | 3.4% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Abbott Laboratories, Terumo Corporation, Cordis, Teleflex Incorporated, Morrris Innovative |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |