What is Global Diclofenac Transdermal Patches Market?
The Global Diclofenac Transdermal Patches Market refers to the worldwide industry focused on the production, distribution, and sale of transdermal patches containing diclofenac, a non-steroidal anti-inflammatory drug (NSAID) used primarily for pain relief and inflammation reduction. These patches are designed to deliver the medication through the skin directly to the affected area, providing a convenient and often more effective alternative to oral medications. The market encompasses various aspects, including research and development, manufacturing processes, regulatory approvals, and marketing strategies. It is influenced by factors such as the prevalence of conditions like arthritis and musculoskeletal pain, advancements in transdermal drug delivery technologies, and consumer preferences for non-invasive treatment options. The market is also shaped by competitive dynamics among pharmaceutical companies, pricing strategies, and the availability of generic alternatives. As healthcare systems globally continue to emphasize patient-centric care and personalized medicine, the demand for such targeted and convenient treatment options is expected to grow, driving further innovation and expansion within this market segment.
12 Hours, 24 Hours in the Global Diclofenac Transdermal Patches Market:
In the Global Diclofenac Transdermal Patches Market, the duration of effectiveness is a crucial factor that influences consumer choice and market dynamics. These patches are typically available in two main variants based on their duration of action: 12-hour and 24-hour patches. The 12-hour patches are designed for individuals who require shorter-term pain relief or prefer to change their patches more frequently. These patches are often favored by patients who experience acute pain episodes or those who are new to transdermal therapy and wish to monitor their body's response to the medication more closely. On the other hand, 24-hour patches offer the convenience of once-daily application, making them ideal for individuals with chronic pain conditions who seek consistent and prolonged relief without the need for frequent reapplication. This variant is particularly beneficial for patients with busy lifestyles or those who may have difficulty remembering to change their patches multiple times a day. The choice between 12-hour and 24-hour patches can also be influenced by factors such as the severity of the condition being treated, patient preference, and healthcare provider recommendations. From a market perspective, the availability of both 12-hour and 24-hour patches allows pharmaceutical companies to cater to a broader range of consumer needs and preferences, thereby enhancing their competitive positioning. Additionally, the development and marketing of these variants require careful consideration of factors such as formulation stability, adhesive properties, and skin compatibility to ensure optimal efficacy and patient satisfaction. As the market continues to evolve, ongoing research and innovation in transdermal drug delivery technologies are expected to further enhance the performance and appeal of both 12-hour and 24-hour diclofenac patches, driving continued growth and diversification within this segment.
Online Sales, Drugstore Sales in the Global Diclofenac Transdermal Patches Market:
The usage of Global Diclofenac Transdermal Patches Market in online sales and drugstore sales represents two distinct yet complementary distribution channels that cater to different consumer preferences and purchasing behaviors. Online sales have gained significant traction in recent years, driven by the increasing adoption of e-commerce platforms and the growing demand for convenient and contactless shopping experiences. Consumers who prefer online shopping often value the ability to compare products, read reviews, and make informed purchasing decisions from the comfort of their homes. For the diclofenac transdermal patches market, online sales offer the advantage of reaching a wider audience, including those in remote or underserved areas where access to physical drugstores may be limited. Additionally, online platforms can facilitate direct-to-consumer sales, allowing manufacturers to engage with customers more directly and gather valuable feedback for product improvement and innovation. On the other hand, drugstore sales remain a vital component of the distribution strategy for diclofenac transdermal patches, particularly for consumers who prefer in-person interactions and the assurance of purchasing from a trusted local pharmacy. Drugstores provide the added benefit of professional guidance from pharmacists, who can offer personalized advice on product selection, usage, and potential interactions with other medications. This channel is especially important for first-time users or those with complex medical needs who may require additional support and reassurance. Furthermore, drugstores often serve as a point of care for patients who need to refill their prescriptions or seek immediate relief from pain and inflammation. The coexistence of online and drugstore sales channels allows the diclofenac transdermal patches market to effectively address the diverse needs and preferences of consumers, ensuring broad accessibility and convenience. As the market continues to grow, companies are likely to invest in strategies that optimize both online and offline sales channels, leveraging digital marketing, customer engagement, and supply chain efficiencies to enhance their competitive edge and drive sustained growth.
Global Diclofenac Transdermal Patches Market Outlook:
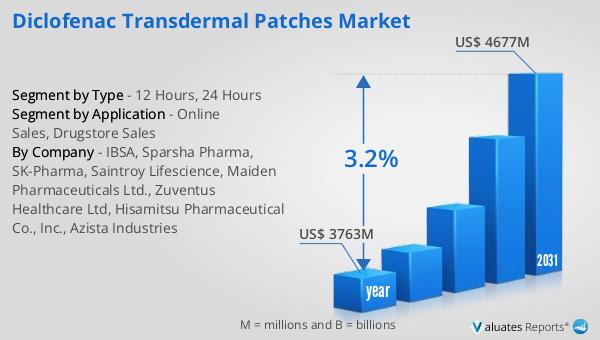
The global market for Diclofenac Transdermal Patches was valued at approximately $3,763 million in 2024, with projections indicating an increase to around $4,677 million by 2031, reflecting a compound annual growth rate (CAGR) of 3.2% over the forecast period. This growth trajectory highlights the expanding demand for transdermal patches as a preferred method of drug delivery, driven by their convenience and effectiveness in managing pain and inflammation. In the broader context of the pharmaceutical industry, the global market was valued at $1,475 billion in 2022, with an anticipated CAGR of 5% over the next six years. This indicates a robust growth outlook for the pharmaceutical sector as a whole, driven by factors such as increasing healthcare expenditure, advancements in drug development, and the rising prevalence of chronic diseases. Comparatively, the chemical drug market, which forms a significant component of the pharmaceutical industry, was estimated to grow from $1,005 billion in 2018 to $1,094 billion in 2022. This growth underscores the ongoing demand for chemical-based therapeutics, including NSAIDs like diclofenac, which continue to play a crucial role in pain management and inflammation control. The interplay between these market segments reflects the dynamic nature of the pharmaceutical landscape, where innovation, regulatory developments, and consumer preferences drive continuous evolution and expansion.
| Report Metric | Details |
| Report Name | Diclofenac Transdermal Patches Market |
| Accounted market size in year | US$ 3763 million |
| Forecasted market size in 2031 | US$ 4677 million |
| CAGR | 3.2% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | IBSA, Sparsha Pharma, SK-Pharma, Saintroy Lifescience, Maiden Pharmaceuticals Ltd., Zuventus Healthcare Ltd, Hisamitsu Pharmaceutical Co., Inc., Azista Industries |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |