What is Global Total Lab Automation System Market?
The Global Total Lab Automation System Market refers to the comprehensive integration of automated technologies in laboratory settings to enhance efficiency, accuracy, and throughput. This market encompasses a wide range of automated instruments and software solutions designed to streamline laboratory processes, reduce human error, and improve data management. Total lab automation systems are increasingly being adopted in various sectors, including clinical diagnostics, pharmaceuticals, biotechnology, and research laboratories. These systems offer numerous benefits, such as faster processing times, improved reproducibility of results, and reduced operational costs. By automating repetitive and time-consuming tasks, laboratories can focus more on complex analyses and research activities. The market is driven by the growing demand for high-throughput screening, advancements in technology, and the need for efficient laboratory workflows. As laboratories continue to face challenges such as increasing sample volumes and the need for precise and reliable results, the adoption of total lab automation systems is expected to rise, making it a crucial component in modern laboratory operations.
Closed, Semi-closed, Open in the Global Total Lab Automation System Market:
In the Global Total Lab Automation System Market, systems are categorized into three main types: Closed, Semi-closed, and Open systems. Closed systems are fully integrated and designed to operate as a single unit, often from a single manufacturer. These systems offer high levels of automation and are typically used in environments where standardization and consistency are critical. They are ideal for high-volume laboratories that require minimal human intervention and maximum efficiency. Closed systems are often preferred for their reliability and ease of use, as they come with pre-configured settings and protocols that ensure consistent results. However, they may lack flexibility, as they are limited to the specific assays and reagents provided by the manufacturer. Semi-closed systems, on the other hand, offer a balance between automation and flexibility. These systems allow for some degree of customization and integration with other laboratory instruments and software. Semi-closed systems are suitable for laboratories that require a certain level of adaptability while still benefiting from the efficiency of automation. They provide the ability to incorporate different assays and reagents from various manufacturers, making them a versatile option for laboratories with diverse testing needs. Semi-closed systems are often used in research and development settings where the ability to modify and optimize protocols is essential. Open systems provide the highest level of flexibility and customization. These systems are designed to integrate seamlessly with a wide range of laboratory instruments and software, allowing laboratories to tailor their workflows to specific needs. Open systems are ideal for laboratories that require a high degree of adaptability and the ability to incorporate new technologies and methodologies as they become available. They are often used in cutting-edge research environments where innovation and experimentation are key. Open systems enable laboratories to optimize their processes and improve efficiency by selecting the best tools and technologies for their specific applications. Each type of system has its advantages and limitations, and the choice between closed, semi-closed, and open systems depends on the specific needs and goals of the laboratory. Closed systems offer simplicity and reliability, making them suitable for high-volume, routine testing environments. Semi-closed systems provide a balance between automation and flexibility, making them ideal for laboratories with diverse testing requirements. Open systems offer the greatest level of customization and are best suited for research and development settings where adaptability and innovation are paramount. As the Global Total Lab Automation System Market continues to evolve, laboratories will need to carefully consider their specific needs and objectives when selecting the appropriate system type to ensure optimal performance and efficiency.
Biochemical Immunity, Blood, Urine in the Global Total Lab Automation System Market:
The Global Total Lab Automation System Market plays a significant role in various laboratory applications, including biochemical immunity, blood, and urine testing. In the field of biochemical immunity, total lab automation systems are used to streamline the analysis of immune responses and the detection of biomarkers associated with various diseases. Automated systems enhance the accuracy and speed of immunoassays, allowing for the rapid identification of antibodies, antigens, and other immune-related molecules. This is particularly important in clinical diagnostics, where timely and precise results are crucial for patient care. By automating these processes, laboratories can handle larger sample volumes and reduce the risk of human error, ultimately improving the quality of diagnostic outcomes. In blood testing, total lab automation systems are employed to automate the analysis of various blood components, such as red and white blood cells, platelets, and plasma. These systems enable laboratories to perform a wide range of tests, including complete blood counts, blood chemistry analyses, and coagulation studies, with greater efficiency and accuracy. Automated blood testing systems reduce the need for manual intervention, minimizing the potential for errors and ensuring consistent results. This is particularly beneficial in high-volume laboratories, where the demand for rapid and reliable blood test results is high. By automating blood testing processes, laboratories can improve turnaround times and enhance the overall quality of patient care. Urine testing is another area where total lab automation systems are making a significant impact. Automated urine analyzers are used to perform routine urinalysis, detecting and quantifying various substances in urine samples, such as proteins, glucose, ketones, and bacteria. These systems offer high throughput and precision, enabling laboratories to process large numbers of samples quickly and accurately. Automated urine testing systems are particularly useful in clinical settings, where timely and accurate results are essential for diagnosing and monitoring various medical conditions, such as urinary tract infections, kidney diseases, and diabetes. By automating urine testing processes, laboratories can improve efficiency, reduce costs, and enhance the overall quality of diagnostic services. Overall, the Global Total Lab Automation System Market is transforming the way laboratories conduct biochemical immunity, blood, and urine testing. By automating these processes, laboratories can achieve higher levels of efficiency, accuracy, and throughput, ultimately improving the quality of diagnostic outcomes and patient care. As the demand for precise and reliable laboratory results continues to grow, the adoption of total lab automation systems is expected to increase, driving further advancements in laboratory technology and practices.
Global Total Lab Automation System Market Outlook:
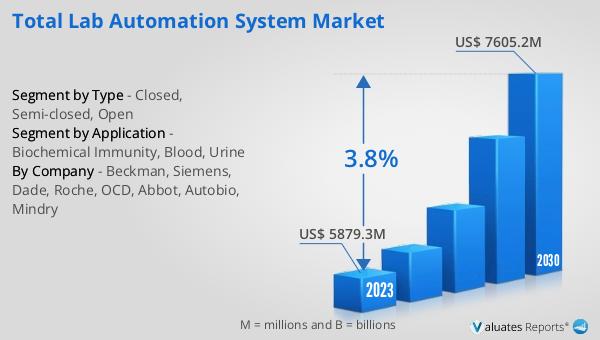
The outlook for the Global Total Lab Automation System Market indicates a promising growth trajectory. The market is anticipated to expand from a valuation of US$ 6080.3 million in 2024 to approximately US$ 7605.2 million by 2030. This growth is expected to occur at a Compound Annual Growth Rate (CAGR) of 3.8% over the forecast period. This steady increase reflects the rising demand for efficient and reliable laboratory automation solutions across various sectors, including clinical diagnostics, pharmaceuticals, and research laboratories. The adoption of total lab automation systems is driven by the need to enhance laboratory workflows, reduce human error, and improve data management. As laboratories face increasing sample volumes and the need for precise and timely results, the integration of automated technologies becomes essential. The market's growth is also supported by advancements in technology, which continue to improve the capabilities and functionalities of lab automation systems. As a result, laboratories are increasingly investing in these systems to optimize their operations and meet the growing demands of modern laboratory practices. The projected growth of the Global Total Lab Automation System Market underscores the importance of automation in enhancing laboratory efficiency and accuracy, ultimately contributing to improved diagnostic outcomes and patient care.
| Report Metric | Details |
| Report Name | Total Lab Automation System Market |
| Accounted market size in 2024 | US$ 6080.3 million |
| Forecasted market size in 2030 | US$ 7605.2 million |
| CAGR | 3.8 |
| Base Year | 2024 |
| Forecasted years | 2025 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Segment by Region |
|
| By Company | Dade, Roche, OCD, Abbot, Autobio, Mindry |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |