What is Nanoparticle Measurement Instrument - Global Market?
Nanoparticle measurement instruments are specialized tools used to analyze and measure nanoparticles, which are particles between 1 and 100 nanometers in size. These instruments are crucial in various fields, including materials science, pharmaceuticals, and environmental science, as they help researchers and industry professionals understand the properties and behaviors of nanoparticles. The global market for these instruments is expanding due to the increasing demand for precise measurement and analysis of nanoparticles in research and industrial applications. As technology advances, these instruments are becoming more sophisticated, offering higher accuracy and efficiency. The market is driven by the need for better quality control, research and development, and regulatory compliance in industries that utilize nanoparticles. The growth of this market is also supported by the rising awareness of the potential health and environmental impacts of nanoparticles, prompting more stringent monitoring and measurement requirements. As a result, the nanoparticle measurement instrument market is poised for significant growth, with innovations and advancements continually enhancing the capabilities and applications of these essential tools.
Handheld, Desktop in the Nanoparticle Measurement Instrument - Global Market:
Handheld and desktop-based nanoparticle measurement instruments are two primary categories within the global market, each serving distinct purposes and offering unique advantages. Handheld instruments are portable and designed for on-the-go measurements, making them ideal for fieldwork and situations where mobility is crucial. These devices are typically user-friendly, allowing for quick and easy measurements without the need for extensive training. They are often used in environmental monitoring, where researchers need to measure nanoparticles in various locations, such as air quality assessments or water testing. The portability of handheld instruments makes them a valuable tool for industries that require immediate data collection and analysis in diverse settings. On the other hand, desktop-based nanoparticle measurement instruments are more robust and offer higher precision and advanced analytical capabilities. These instruments are typically used in laboratory settings where detailed and accurate measurements are required. They are equipped with sophisticated software and hardware that allow for comprehensive analysis of nanoparticle size, distribution, and concentration. Desktop instruments are essential in research and development, quality control, and regulatory compliance, providing detailed insights into the properties and behaviors of nanoparticles. The choice between handheld and desktop instruments depends on the specific needs and requirements of the user. Handheld instruments offer convenience and flexibility, while desktop instruments provide precision and depth of analysis. As the demand for nanoparticle measurement continues to grow, both types of instruments are expected to see increased adoption across various industries. The development of new technologies and innovations in this field is likely to enhance the capabilities of both handheld and desktop instruments, making them even more valuable tools for researchers and industry professionals. The global market for these instruments is driven by the need for accurate and reliable nanoparticle measurement, with advancements in technology continually pushing the boundaries of what is possible. As industries become more reliant on nanoparticles, the demand for effective measurement tools will continue to rise, supporting the growth of the nanoparticle measurement instrument market.
Environmental Monitoring, Industrial Emission Control, Others in the Nanoparticle Measurement Instrument - Global Market:
Nanoparticle measurement instruments play a crucial role in environmental monitoring, industrial emission control, and other applications. In environmental monitoring, these instruments are used to measure the concentration and distribution of nanoparticles in the air, water, and soil. This information is vital for assessing the impact of nanoparticles on the environment and human health. By providing accurate and reliable data, nanoparticle measurement instruments help researchers and policymakers develop strategies to mitigate the potential risks associated with nanoparticles. In industrial emission control, these instruments are used to monitor and regulate the release of nanoparticles from industrial processes. Industries such as manufacturing, energy production, and waste management often produce nanoparticles as byproducts, which can have harmful effects on the environment and human health if not properly controlled. Nanoparticle measurement instruments enable industries to comply with regulatory standards and implement effective emission control measures. In addition to environmental monitoring and industrial emission control, nanoparticle measurement instruments are used in various other applications, including pharmaceuticals, materials science, and biotechnology. In the pharmaceutical industry, these instruments are used to analyze the size and distribution of nanoparticles in drug formulations, ensuring the safety and efficacy of nanoparticle-based therapies. In materials science, nanoparticle measurement instruments are used to study the properties and behaviors of nanoparticles, leading to the development of new materials with enhanced performance and functionality. In biotechnology, these instruments are used to measure nanoparticles in biological samples, providing insights into their interactions with biological systems. The versatility and precision of nanoparticle measurement instruments make them indispensable tools in a wide range of applications, driving their demand and supporting the growth of the global market. As the understanding of nanoparticles and their potential impacts continues to evolve, the need for accurate and reliable measurement tools will become increasingly important, further fueling the growth of the nanoparticle measurement instrument market.
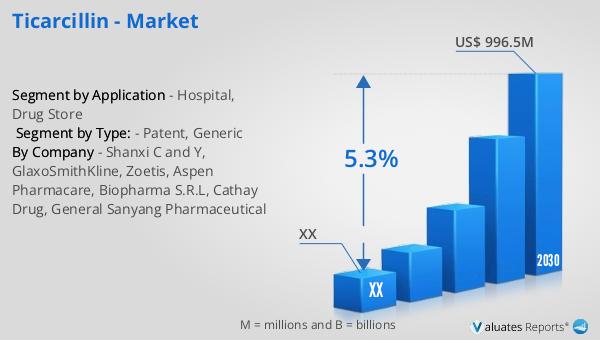
Nanoparticle Measurement Instrument - Global Market Outlook:
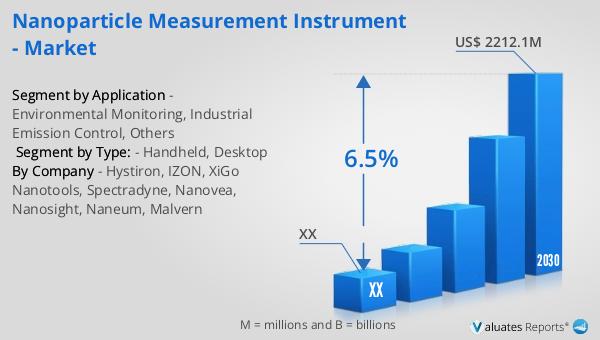
The global market for nanoparticle measurement instruments was valued at approximately $1,426 million in 2023. It is projected to grow to a revised size of $2,212.1 million by 2030, reflecting a compound annual growth rate (CAGR) of 6.5% during the forecast period from 2024 to 2030. This growth is indicative of the increasing importance and demand for these instruments across various industries. The laboratory analyzer segment, a high-value, technology-intensive area within the instrumentation industry, has seen rapid technological advancements in recent years. This has contributed to the positive momentum and expansion of the market size. The development of new technologies and innovations in nanoparticle measurement instruments is enhancing their capabilities and applications, making them more valuable tools for researchers and industry professionals. As industries become more reliant on nanoparticles, the demand for effective measurement tools will continue to rise, supporting the growth of the nanoparticle measurement instrument market. The increasing awareness of the potential health and environmental impacts of nanoparticles is also driving the demand for these instruments, as more stringent monitoring and measurement requirements are implemented. Overall, the global market for nanoparticle measurement instruments is poised for significant growth, with advancements in technology continually pushing the boundaries of what is possible.
| Report Metric | Details |
| Report Name | Nanoparticle Measurement Instrument - Market |
| Forecasted market size in 2030 | US$ 2212.1 million |
| CAGR | 6.5% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Hystiron, IZON, XiGo Nanotools, Spectradyne, Nanovea, Nanosight, Naneum, Malvern |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |