What is Artificial Plasma - Global Market?
Artificial plasma is a fascinating and innovative area within the global market, primarily focused on developing synthetic alternatives to natural blood plasma. This market is driven by the need for safe, effective, and readily available blood substitutes, especially in situations where natural blood supply is limited or risky. Artificial plasma products are designed to mimic the functions of natural plasma, such as transporting nutrients, hormones, and proteins throughout the body, while also maintaining blood pressure and volume. The global market for artificial plasma is expanding due to advancements in biotechnology and increasing demand from healthcare sectors worldwide. These products are particularly valuable in emergency medicine, surgery, and trauma care, where rapid blood loss can occur, and immediate plasma replacement is critical. The development of artificial plasma also addresses concerns related to blood-borne diseases and compatibility issues, offering a safer alternative for patients requiring transfusions. As research and technology continue to evolve, the artificial plasma market is poised to play a significant role in the future of medical treatments and emergency care.
HBOC, PFBOC in the Artificial Plasma - Global Market:
HBOC (Hemoglobin-Based Oxygen Carriers) and PFBOC (Perfluorocarbon-Based Oxygen Carriers) are two prominent types of artificial plasma products that have gained attention in the global market. HBOCs are designed to transport oxygen throughout the body, similar to the function of hemoglobin in red blood cells. These products are particularly useful in situations where blood transfusions are not possible or practical, such as in remote locations or during military operations. HBOCs are engineered to be compatible with all blood types, reducing the risk of transfusion reactions and eliminating the need for blood typing and cross-matching. This makes them an attractive option for emergency medical situations where time is of the essence. PFBOCs, on the other hand, are synthetic compounds that can dissolve large amounts of oxygen and carbon dioxide, making them effective oxygen carriers. They are particularly useful in situations where oxygen delivery is compromised, such as in cases of severe anemia or respiratory distress. PFBOCs are also being explored for use in organ preservation, as they can help maintain oxygenation during transport and storage. Both HBOCs and PFBOCs offer significant advantages over traditional blood products, including longer shelf life, reduced risk of contamination, and the ability to be produced in large quantities. However, there are also challenges associated with their use, including potential side effects and the need for further research to fully understand their long-term effects on the body. Despite these challenges, the global market for HBOCs and PFBOCs is expected to grow as demand for safe and effective blood substitutes continues to rise. As technology advances and more research is conducted, these products have the potential to revolutionize the way we approach blood transfusions and oxygen delivery in medical settings.
Human Use, Veterinary Use in the Artificial Plasma - Global Market:
The usage of artificial plasma in the global market extends to both human and veterinary applications, offering a wide range of benefits in various medical scenarios. For human use, artificial plasma products are primarily utilized in emergency medicine, surgery, and trauma care, where rapid blood loss can occur, and immediate plasma replacement is critical. These products provide a safe and effective alternative to natural blood plasma, reducing the risk of transfusion reactions and blood-borne diseases. In addition to emergency situations, artificial plasma is also being explored for use in chronic conditions, such as anemia and certain types of cancer, where regular blood transfusions are required. The ability to produce artificial plasma in large quantities and with a longer shelf life than natural plasma makes it an attractive option for healthcare providers looking to improve patient outcomes and reduce costs. In the veterinary field, artificial plasma is used to treat a variety of conditions in animals, including trauma, surgery, and chronic illnesses. Similar to human applications, artificial plasma provides a safe and effective alternative to natural blood products, reducing the risk of transfusion reactions and disease transmission. Veterinary use of artificial plasma is particularly valuable in situations where natural blood supply is limited or unavailable, such as in remote locations or during emergencies. The ability to produce artificial plasma in large quantities and with a longer shelf life than natural plasma makes it an attractive option for veterinarians looking to improve animal health and welfare. As research and technology continue to advance, the use of artificial plasma in both human and veterinary medicine is expected to grow, offering new opportunities for improving patient care and outcomes.
Artificial Plasma - Global Market Outlook:
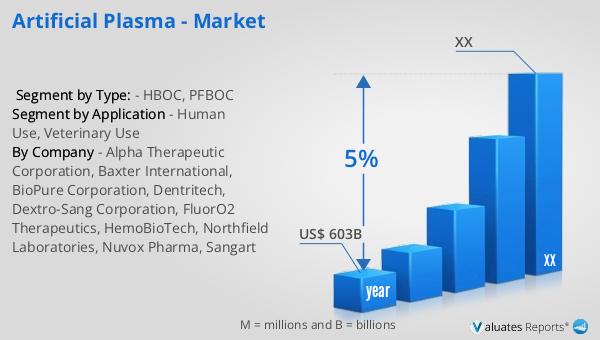
Our research indicates that the global market for medical devices, which includes artificial plasma products, is valued at approximately US$ 603 billion in 2023. This market is projected to experience a compound annual growth rate (CAGR) of 5% over the next six years. This growth is driven by several factors, including advancements in medical technology, increasing demand for innovative healthcare solutions, and the rising prevalence of chronic diseases that require ongoing medical intervention. The development and adoption of artificial plasma products are expected to contribute significantly to this growth, as they offer a safe and effective alternative to traditional blood products. The ability to produce artificial plasma in large quantities and with a longer shelf life than natural plasma makes it an attractive option for healthcare providers looking to improve patient outcomes and reduce costs. Additionally, the global market for artificial plasma is expected to benefit from increasing investments in research and development, as well as growing awareness of the benefits of these products among healthcare professionals and patients. As the market continues to expand, artificial plasma products are poised to play a significant role in the future of medical treatments and emergency care, offering new opportunities for improving patient outcomes and reducing healthcare costs.
| Report Metric | Details |
| Report Name | Artificial Plasma - Market |
| Accounted market size in year | US$ 603 billion |
| CAGR | 5% |
| Base Year | year |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Alpha Therapeutic Corporation, Baxter International, BioPure Corporation, Dentritech, Dextro-Sang Corporation, FluorO2 Therapeutics, HemoBioTech, Northfield Laboratories, Nuvox Pharma, Sangart |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |