What is Global Rituximab Biosimilars Market?
The Global Rituximab Biosimilars Market refers to the worldwide industry focused on the development, production, and distribution of biosimilar versions of Rituximab, a monoclonal antibody used primarily in the treatment of certain autoimmune diseases and types of cancer, such as non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Biosimilars are essentially biologic medical products that are highly similar to already approved reference products, with no significant differences in terms of safety, purity, and potency. The market for Rituximab biosimilars is driven by the increasing prevalence of cancer and autoimmune diseases, the rising demand for cost-effective treatment options, and the expiration of patents for original biologic drugs, which opens the door for biosimilar competition. As healthcare systems worldwide face pressure to reduce costs while maintaining high standards of care, biosimilars offer a promising solution by providing more affordable alternatives to expensive biologic therapies. The market is characterized by intense competition among pharmaceutical companies, regulatory challenges, and the need for extensive clinical trials to ensure biosimilarity and safety. Overall, the Global Rituximab Biosimilars Market plays a crucial role in expanding access to essential medications and supporting the sustainability of healthcare systems globally.
500mg, 100mg, Other in the Global Rituximab Biosimilars Market:
In the Global Rituximab Biosimilars Market, the products are typically available in different dosage forms, including 500mg, 100mg, and other variations, to cater to diverse patient needs and treatment protocols. The 500mg dosage form is commonly used in clinical settings for the treatment of various conditions, including non-Hodgkin's lymphoma and rheumatoid arthritis. This higher dosage is often administered to patients who require a more substantial amount of the drug to achieve therapeutic efficacy, particularly in cases where the disease is more advanced or aggressive. The 500mg dosage form is typically delivered via intravenous infusion, allowing for controlled administration and optimal absorption of the medication into the bloodstream. On the other hand, the 100mg dosage form is often used for patients who require a lower dose of the medication, either due to the nature of their condition or as part of a maintenance therapy regimen. This dosage form is also administered intravenously and is particularly useful in situations where precise dosing is critical to avoid potential side effects or complications. In addition to the standard 500mg and 100mg dosage forms, the market also includes other variations that may be tailored to specific patient populations or treatment protocols. These variations can include different concentrations, formulations, or delivery mechanisms designed to enhance the convenience, efficacy, or safety of the medication. For instance, some biosimilars may be available in pre-filled syringes or vials, offering healthcare providers greater flexibility in administering the drug and reducing the risk of dosing errors. The availability of multiple dosage forms and variations in the Global Rituximab Biosimilars Market reflects the industry's commitment to meeting the diverse needs of patients and healthcare providers. By offering a range of options, pharmaceutical companies can ensure that patients receive the most appropriate and effective treatment for their specific condition, while also addressing practical considerations such as ease of administration and cost-effectiveness. This diversity in product offerings is particularly important in the context of personalized medicine, where treatment plans are increasingly tailored to the individual characteristics and preferences of each patient. Overall, the availability of different dosage forms and variations in the Global Rituximab Biosimilars Market underscores the importance of flexibility and innovation in the development and delivery of biosimilar therapies. By providing a wide range of options, the market can better serve the needs of patients and healthcare providers, ultimately contributing to improved health outcomes and greater access to life-saving medications.
Hospital Pharmacy, Retail Pharmacy in the Global Rituximab Biosimilars Market:
The usage of Rituximab biosimilars in hospital and retail pharmacies plays a significant role in the broader healthcare landscape, offering both economic and therapeutic benefits. In hospital pharmacies, Rituximab biosimilars are primarily used to treat patients with conditions such as non-Hodgkin's lymphoma, chronic lymphocytic leukemia, and rheumatoid arthritis. These biosimilars provide a cost-effective alternative to the original biologic drugs, enabling hospitals to manage their budgets more efficiently while still delivering high-quality care. The availability of Rituximab biosimilars in hospital settings also allows for greater flexibility in treatment planning, as healthcare providers can choose from a range of biosimilar options to best meet the needs of their patients. This flexibility is particularly important in the context of personalized medicine, where treatment plans are increasingly tailored to the individual characteristics and preferences of each patient. In retail pharmacies, Rituximab biosimilars are typically dispensed to patients who require ongoing treatment for chronic conditions. The availability of these biosimilars in retail settings ensures that patients have convenient access to their medications, reducing the need for frequent hospital visits and allowing for greater continuity of care. Retail pharmacies also play a crucial role in educating patients about the use of biosimilars, providing information on dosing, administration, and potential side effects. This patient education is essential for ensuring that biosimilars are used safely and effectively, ultimately contributing to improved health outcomes. The use of Rituximab biosimilars in both hospital and retail pharmacies also has broader implications for the healthcare system as a whole. By providing more affordable treatment options, biosimilars can help to reduce overall healthcare costs, freeing up resources that can be reinvested in other areas of patient care. This cost savings is particularly important in the context of rising healthcare expenditures and the increasing demand for healthcare services. Furthermore, the use of biosimilars can help to increase competition in the pharmaceutical market, driving innovation and encouraging the development of new and improved therapies. Overall, the usage of Rituximab biosimilars in hospital and retail pharmacies represents a significant advancement in the delivery of healthcare, offering both economic and therapeutic benefits to patients and healthcare providers alike. By providing more affordable and accessible treatment options, biosimilars can help to improve health outcomes, reduce healthcare costs, and support the sustainability of healthcare systems worldwide.
Global Rituximab Biosimilars Market Outlook:
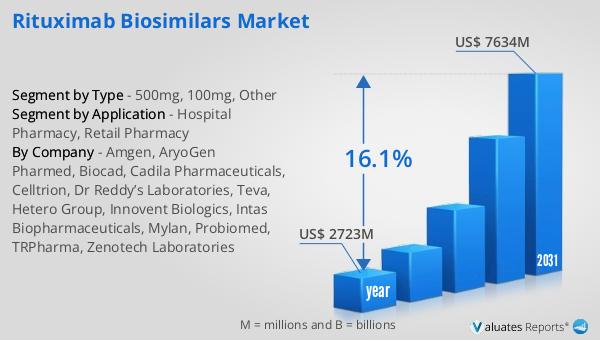
The global market for Rituximab Biosimilars was valued at $2,723 million in 2024 and is anticipated to expand to a revised size of $7,634 million by 2031, reflecting a robust compound annual growth rate (CAGR) of 16.1% over the forecast period. This impressive growth trajectory underscores the increasing demand for biosimilar products as cost-effective alternatives to traditional biologic therapies. In contrast, the global pharmaceutical market was valued at $1,475 billion in 2022, with a more modest growth rate of 5% projected over the next six years. This comparison highlights the rapid expansion of the biosimilars sector within the broader pharmaceutical industry, driven by factors such as patent expirations, rising healthcare costs, and the growing prevalence of chronic diseases. Meanwhile, the chemical drug market, which was valued at $1,005 billion in 2018, is estimated to have grown to $1,094 billion by 2022. This slower growth rate reflects the maturity of the chemical drug sector and the increasing shift towards biologic and biosimilar therapies. Overall, the Rituximab Biosimilars Market is poised for significant growth, driven by the need for more affordable and accessible treatment options in the face of rising healthcare demands.
| Report Metric | Details |
| Report Name | Rituximab Biosimilars Market |
| Accounted market size in year | US$ 2723 million |
| Forecasted market size in 2031 | US$ 7634 million |
| CAGR | 16.1% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Amgen, AryoGen Pharmed, Biocad, Cadila Pharmaceuticals, Celltrion, Dr Reddy’s Laboratories, Teva, Hetero Group, Innovent Biologics, Intas Biopharmaceuticals, Mylan, Probiomed, TRPharma, Zenotech Laboratories |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
