What is Monkeypox Virus Detection Reagent - Global Market?
The Monkeypox Virus Detection Reagent is a crucial component in the global market for diagnosing and managing monkeypox, a viral zoonotic disease that has gained attention due to its potential to cause outbreaks. This reagent is used in various diagnostic tests to identify the presence of the monkeypox virus in human samples. The global market for these detection reagents is driven by the increasing need for accurate and rapid diagnosis, especially in regions where monkeypox is endemic or where outbreaks have occurred. The market encompasses a range of products, including PCR kits, serological assays, and other molecular diagnostic tools that are designed to detect the virus with high specificity and sensitivity. As awareness of monkeypox grows and the demand for effective diagnostic solutions increases, the market for these reagents is expected to expand significantly. This growth is further supported by advancements in diagnostic technologies and the increasing availability of these reagents in various healthcare settings worldwide. The market's expansion is also influenced by collaborations between governments, healthcare organizations, and diagnostic companies to enhance the accessibility and affordability of monkeypox testing. Overall, the Monkeypox Virus Detection Reagent market plays a vital role in the global effort to control and prevent the spread of monkeypox.
Vesicles or Pustules, Nasopharyngeal Swab, Oropharyngeal Swab, Serum, Whole Blood in the Monkeypox Virus Detection Reagent - Global Market:
In the context of the Monkeypox Virus Detection Reagent - Global Market, various biological samples are utilized to detect the presence of the virus, each offering unique advantages and challenges. Vesicles or pustules, which are fluid-filled lesions that appear on the skin of infected individuals, are among the primary samples used for monkeypox virus detection. These lesions contain a high concentration of the virus, making them ideal for direct testing. Swabbing these vesicles allows for the collection of viral DNA, which can then be analyzed using PCR (Polymerase Chain Reaction) techniques to confirm the presence of the virus. Nasopharyngeal swabs are another critical sample type used in the detection of monkeypox. These swabs are collected from the upper part of the throat behind the nose and are particularly useful in the early stages of infection when respiratory symptoms may be present. The nasopharyngeal swab is inserted through the nostril and gently rotated to collect cells and secretions, which are then tested for viral genetic material. This method is non-invasive and can be performed quickly, making it a practical choice for widespread testing. Oropharyngeal swabs, collected from the back of the throat, are also used in monkeypox virus detection. Similar to nasopharyngeal swabs, these samples are valuable for identifying the virus in the respiratory tract, especially in cases where respiratory symptoms are prominent. The collection process involves swabbing the tonsillar area and the posterior pharynx, ensuring that sufficient material is gathered for accurate testing. Serum samples, which are derived from the liquid portion of blood after clotting, are used in serological assays to detect antibodies against the monkeypox virus. These tests are crucial for understanding the immune response in infected individuals and can provide insights into the spread of the virus within a population. Whole blood samples, on the other hand, are used for both serological and molecular testing. They offer a comprehensive view of the infection status and can be used to detect both viral DNA and antibodies. The choice of sample type in monkeypox virus detection depends on various factors, including the stage of infection, the availability of testing resources, and the specific diagnostic needs. Each sample type has its own set of advantages and limitations, and the selection often involves a balance between accuracy, invasiveness, and practicality. As the global market for monkeypox virus detection reagents continues to grow, the development of more efficient and user-friendly sampling methods is likely to enhance the overall testing process, making it more accessible and reliable for healthcare providers worldwide.
Hospital, Clinic in the Monkeypox Virus Detection Reagent - Global Market:
The usage of Monkeypox Virus Detection Reagents in hospitals and clinics is pivotal in managing and controlling the spread of the disease. In hospital settings, these reagents are integral to the diagnostic process, enabling healthcare professionals to quickly and accurately identify monkeypox cases. Hospitals, often equipped with advanced laboratory facilities, utilize these reagents in conjunction with sophisticated diagnostic equipment to perform tests such as PCR and serological assays. The ability to rapidly diagnose monkeypox in a hospital environment is crucial, as it allows for timely isolation and treatment of infected patients, thereby reducing the risk of transmission within the facility and the broader community. Moreover, hospitals play a key role in monitoring and managing outbreaks, and the availability of reliable detection reagents is essential for effective surveillance and response efforts. In clinics, the use of Monkeypox Virus Detection Reagents is equally important, albeit on a smaller scale compared to hospitals. Clinics serve as the first point of contact for many patients, and the ability to conduct on-site testing is invaluable for early detection and intervention. While clinics may not have the same level of laboratory infrastructure as hospitals, the development of portable and easy-to-use diagnostic kits has made it possible for these facilities to perform accurate testing. This accessibility is particularly important in rural or underserved areas where healthcare resources may be limited. By providing timely and accurate diagnosis, clinics can initiate appropriate treatment plans and refer patients to higher-level care if necessary. The integration of monkeypox detection reagents into both hospital and clinic settings underscores the importance of a coordinated healthcare approach in managing infectious diseases. As the global market for these reagents expands, it is expected that their availability and affordability will improve, further enhancing the capacity of healthcare systems to respond to monkeypox outbreaks. This expansion is supported by ongoing research and development efforts aimed at improving the sensitivity, specificity, and ease of use of diagnostic tests. Ultimately, the widespread use of Monkeypox Virus Detection Reagents in hospitals and clinics is a critical component of public health strategies aimed at controlling the spread of monkeypox and protecting vulnerable populations.
Monkeypox Virus Detection Reagent - Global Market Outlook:
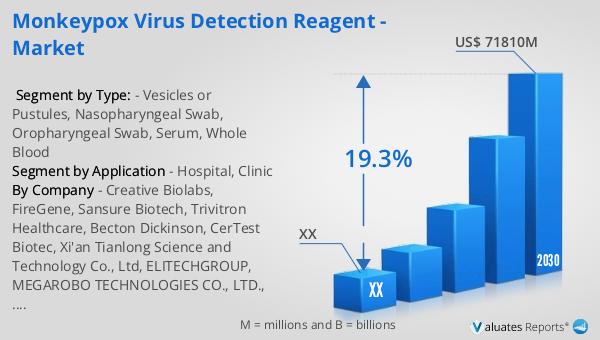
The global market for Monkeypox Virus Detection Reagents was valued at approximately USD 20,670 million in 2023. It is projected to grow significantly, reaching an estimated size of USD 71,810 million by 2030, with a compound annual growth rate (CAGR) of 19.3% during the forecast period from 2024 to 2030. This substantial growth reflects the increasing demand for effective diagnostic solutions in response to the rising incidence of monkeypox cases worldwide. The North American market, in particular, is expected to experience notable growth, although specific figures for this region were not provided. The anticipated expansion of the market is driven by several factors, including advancements in diagnostic technologies, increased awareness of monkeypox, and the need for rapid and accurate testing methods. Additionally, collaborations between governments, healthcare organizations, and diagnostic companies are likely to enhance the accessibility and affordability of monkeypox testing, further contributing to market growth. As the market evolves, it is expected that new and innovative detection reagents will be developed, offering improved sensitivity and specificity for monkeypox diagnosis. This growth trajectory underscores the critical role of Monkeypox Virus Detection Reagents in global efforts to control and prevent the spread of this infectious disease.
| Report Metric | Details |
| Report Name | Monkeypox Virus Detection Reagent - Market |
| Forecasted market size in 2030 | US$ 71810 million |
| CAGR | 19.3% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Creative Biolabs, FireGene, Sansure Biotech, Trivitron Healthcare, Becton Dickinson, CerTest Biotec, Xi'an Tianlong Science and Technology Co., Ltd, ELITECHGROUP, MEGAROBO TECHNOLOGIES CO., LTD., Jiangsu Mole Bioscience CO., LTD., Jiangsu Macro & Micro-Test Med-Tech Co., Ltd., BEIJING APPLIED BIOLOGICAL TECHNOLOGIES, Wuhan J.H.Bio-Tech, Goldsite Diagnostics Inc. |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
