What is Global Urology Guidewires Market?
The Global Urology Guidewires Market is a specialized segment within the broader medical device industry, focusing on the production and distribution of guidewires used in urological procedures. Urology guidewires are essential tools in minimally invasive surgeries and diagnostic procedures, aiding in the navigation and placement of catheters and other medical instruments within the urinary tract. These guidewires are designed to be flexible yet sturdy, allowing for precise control and maneuverability during procedures. The market is driven by the increasing prevalence of urological disorders such as kidney stones, urinary tract infections, and prostate issues, which necessitate surgical intervention. Technological advancements in guidewire materials and design have also contributed to market growth, offering improved patient outcomes and procedural efficiency. Additionally, the rising demand for minimally invasive surgical techniques, which reduce recovery time and hospital stays, further propels the market. The global urology guidewires market is characterized by a diverse range of products, catering to various procedural needs and patient demographics, making it a vital component of modern urological care.
Steel-based Urology Guidewires, Nitinol-based Urology Giudewires in the Global Urology Guidewires Market:
Steel-based urology guidewires are a significant category within the Global Urology Guidewires Market, known for their robustness and reliability. These guidewires are typically made from stainless steel, which provides excellent tensile strength and resistance to kinking. This makes them particularly suitable for procedures that require a high degree of precision and control, such as navigating through complex anatomical structures or accessing hard-to-reach areas within the urinary tract. The rigidity of steel-based guidewires allows for better torque control, which is crucial in procedures like ureteroscopy or percutaneous nephrolithotomy, where precise navigation is essential. However, the stiffness of steel can sometimes be a limitation, as it may increase the risk of trauma to delicate tissues. To mitigate this, many steel-based guidewires are coated with hydrophilic or other specialized coatings to enhance their lubricity and reduce friction during insertion and manipulation. On the other hand, nitinol-based urology guidewires offer a different set of advantages, primarily due to the unique properties of nitinol, a nickel-titanium alloy. Nitinol is renowned for its superelasticity and shape memory, which allow the guidewire to bend and flex without losing its original shape. This makes nitinol-based guidewires highly adaptable to the dynamic and often tortuous pathways of the urinary tract. Their flexibility reduces the risk of tissue damage and enhances patient safety, making them a preferred choice for delicate procedures. Additionally, nitinol guidewires can be engineered to have varying degrees of stiffness along their length, providing a balance between flexibility and support where needed. This versatility makes them suitable for a wide range of urological applications, from simple catheter placements to complex stone retrieval procedures. The choice between steel-based and nitinol-based guidewires often depends on the specific requirements of the procedure, the patient's anatomy, and the surgeon's preference. Both types of guidewires have their own set of benefits and limitations, and ongoing research and development continue to enhance their performance and expand their applications in urological care. As the Global Urology Guidewires Market evolves, the demand for both steel-based and nitinol-based guidewires is expected to grow, driven by advancements in material science and a deeper understanding of urological pathologies.
Hospitals, Clinics, ASCs in the Global Urology Guidewires Market:
The usage of Global Urology Guidewires Market products is prevalent across various healthcare settings, including hospitals, clinics, and ambulatory surgical centers (ASCs), each offering unique advantages and challenges. In hospitals, urology guidewires are integral to a wide range of procedures, from routine catheterizations to complex surgeries like ureteral stent placements and stone extractions. Hospitals often have the infrastructure and resources to handle high volumes of patients and complex cases, making them a primary setting for the use of advanced guidewire technologies. The availability of multidisciplinary teams and state-of-the-art equipment in hospitals further enhances the effectiveness of urology guidewires, allowing for comprehensive patient care and improved outcomes. In clinics, the use of urology guidewires is typically focused on diagnostic and minor therapeutic procedures. Clinics offer a more accessible and cost-effective option for patients, providing timely interventions without the need for hospitalization. The portability and ease of use of modern guidewires make them well-suited for the clinic environment, where quick and efficient procedures are often prioritized. Clinics also play a crucial role in follow-up care and monitoring of patients with chronic urological conditions, utilizing guidewires for routine assessments and interventions. Ambulatory surgical centers (ASCs) represent a growing segment in the healthcare landscape, offering a middle ground between hospitals and clinics. ASCs are designed to provide surgical care that does not require an overnight hospital stay, making them ideal for minimally invasive urological procedures. The use of urology guidewires in ASCs is driven by the demand for efficient, cost-effective surgical solutions that prioritize patient comfort and quick recovery. ASCs often focus on high-volume, low-complexity procedures, where the precision and reliability of guidewires are critical to success. The streamlined operations and specialized focus of ASCs enable them to deliver high-quality care with reduced waiting times and lower costs compared to traditional hospital settings. Across all these settings, the Global Urology Guidewires Market continues to innovate and adapt, providing healthcare professionals with the tools they need to deliver effective and patient-centered care. The versatility and adaptability of urology guidewires make them indispensable in the management of a wide range of urological conditions, contributing to better patient outcomes and enhanced quality of life.
Global Urology Guidewires Market Outlook:
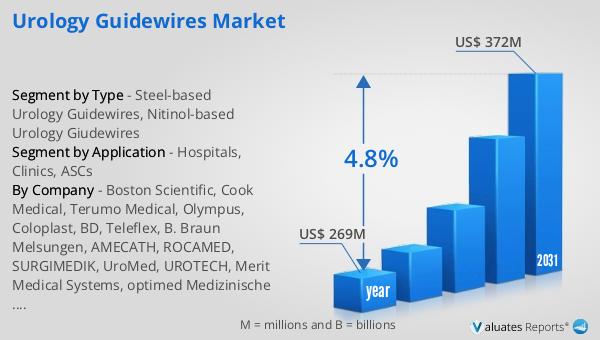
The global market for urology guidewires, valued at $269 million in 2024, is anticipated to expand significantly, reaching an estimated $372 million by 2031. This growth trajectory reflects a compound annual growth rate (CAGR) of 4.8% over the forecast period. This upward trend is indicative of the increasing demand for urology guidewires, driven by factors such as the rising prevalence of urological disorders and the growing adoption of minimally invasive surgical techniques. The market's expansion is also supported by continuous advancements in guidewire technology, which enhance procedural efficiency and patient outcomes. As healthcare systems worldwide strive to improve the quality of care and reduce costs, the role of urology guidewires in facilitating effective and efficient urological procedures becomes increasingly important. The projected growth of the market underscores the critical role that these devices play in modern healthcare, addressing the needs of both patients and healthcare providers. As the market evolves, it is expected to continue adapting to the changing landscape of urological care, offering innovative solutions that meet the diverse needs of the global population. The sustained growth of the Global Urology Guidewires Market highlights its significance as a key component of the medical device industry, contributing to the advancement of urological care worldwide.
| Report Metric | Details |
| Report Name | Urology Guidewires Market |
| Accounted market size in year | US$ 269 million |
| Forecasted market size in 2031 | US$ 372 million |
| CAGR | 4.8% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Boston Scientific, Cook Medical, Terumo Medical, Olympus, Coloplast, BD, Teleflex, B. Braun Melsungen, AMECATH, ROCAMED, SURGIMEDIK, UroMed, UROTECH, Merit Medical Systems, optimed Medizinische Instrumente, MICRO-TECH (Nanjing), Jiangsu Changmei Medtech, Innovex Group, Jiangsu Vedkang Medical, MicroPort, Beyomed, Hunan Ruibang Medical, Hangzhou AGS Medical Technology |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
