What is Global In Vitro Diagnostics (IVD) Quality Control Product Market?
The Global In Vitro Diagnostics (IVD) Quality Control Product Market is a crucial segment within the healthcare industry, focusing on ensuring the accuracy and reliability of diagnostic tests conducted outside the human body. These diagnostics are essential for detecting diseases, monitoring health conditions, and guiding treatment decisions. Quality control products in this market are designed to validate the performance of IVD tests, ensuring they produce consistent and accurate results. This market encompasses a wide range of products, including control materials, calibrators, and proficiency testing tools, which are used by laboratories and healthcare providers worldwide. As the demand for precise and reliable diagnostic tests increases, driven by factors such as the rising prevalence of chronic diseases and the growing emphasis on personalized medicine, the IVD quality control product market is expected to expand. This growth is further supported by technological advancements in diagnostic testing and the increasing adoption of automated laboratory systems. Overall, the Global IVD Quality Control Product Market plays a vital role in enhancing the quality and reliability of diagnostic testing, ultimately contributing to improved patient outcomes and healthcare efficiency.
Quality Control Products, Quality Assurance Services, Data Management Solutions in the Global In Vitro Diagnostics (IVD) Quality Control Product Market:
Quality control products, quality assurance services, and data management solutions are integral components of the Global In Vitro Diagnostics (IVD) Quality Control Product Market. Quality control products are essential for verifying the accuracy and precision of diagnostic tests. These products include control materials, which are used to assess the performance of IVD tests by providing known values against which test results can be compared. Calibrators are another type of quality control product, used to adjust the output of diagnostic instruments to ensure they produce accurate results. Proficiency testing tools are also crucial, allowing laboratories to evaluate their testing capabilities by comparing their results with those of other labs. Quality assurance services complement these products by providing comprehensive support to laboratories in maintaining high standards of testing accuracy and reliability. These services include regular audits, training programs, and consultancy services to help labs implement best practices and comply with regulatory requirements. Data management solutions are increasingly important in the IVD quality control market, as they enable laboratories to efficiently manage and analyze large volumes of test data. These solutions include software platforms that facilitate data integration, storage, and analysis, helping labs to streamline their operations and improve decision-making. By leveraging these solutions, laboratories can enhance their testing efficiency, reduce errors, and ensure compliance with industry standards. Overall, the combination of quality control products, quality assurance services, and data management solutions plays a critical role in ensuring the accuracy and reliability of IVD tests, ultimately contributing to improved patient care and healthcare outcomes.
Clinical Chemistry, Immunochemistry, Hematology, Molecular Diagnostics, Others in the Global In Vitro Diagnostics (IVD) Quality Control Product Market:
The Global In Vitro Diagnostics (IVD) Quality Control Product Market finds extensive application across various areas of clinical testing, including clinical chemistry, immunochemistry, hematology, molecular diagnostics, and others. In clinical chemistry, quality control products are used to ensure the accuracy of tests that measure chemical components in body fluids, such as glucose, cholesterol, and electrolytes. These tests are vital for diagnosing and monitoring conditions like diabetes, cardiovascular diseases, and kidney disorders. In immunochemistry, quality control products help validate tests that detect and quantify proteins, hormones, and other molecules involved in immune responses. These tests are crucial for diagnosing autoimmune diseases, allergies, and infectious diseases. In hematology, quality control products are used to ensure the accuracy of tests that analyze blood cells and coagulation factors, which are essential for diagnosing blood disorders like anemia, leukemia, and clotting disorders. Molecular diagnostics is another key area where quality control products play a vital role. These products are used to validate tests that detect genetic material, such as DNA and RNA, for diagnosing infectious diseases, genetic disorders, and cancer. Other areas where IVD quality control products are used include microbiology, where they help ensure the accuracy of tests that identify and characterize microorganisms, and point-of-care testing, where they validate tests conducted outside traditional laboratory settings. Overall, the use of quality control products across these diverse areas of clinical testing is essential for ensuring the accuracy and reliability of diagnostic results, ultimately contributing to improved patient care and healthcare outcomes.
Global In Vitro Diagnostics (IVD) Quality Control Product Market Outlook:
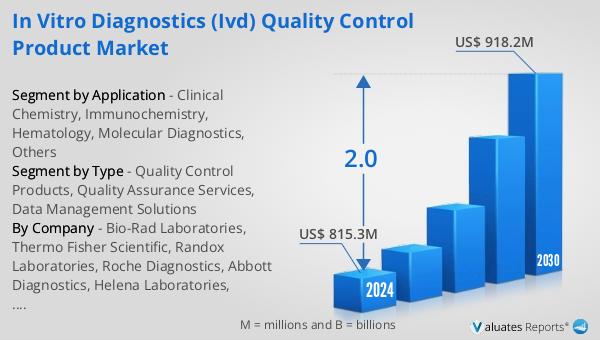
The outlook for the Global In Vitro Diagnostics (IVD) Quality Control Product Market indicates a steady growth trajectory. The market is anticipated to expand from $815.3 million in 2024 to $918.2 million by 2030, reflecting a compound annual growth rate (CAGR) of 2.0% over the forecast period. This growth is driven by the increasing demand for accurate and reliable diagnostic tests, which are essential for effective disease management and treatment. In comparison, the global pharmaceutical market, valued at $1,475 billion in 2022, is projected to grow at a CAGR of 5% over the next six years. This highlights the significant role of the IVD quality control product market within the broader healthcare industry. Additionally, the chemical drug market is expected to grow from $1,005 billion in 2018 to $1,094 billion in 2022, underscoring the ongoing demand for pharmaceutical products. The steady growth of the IVD quality control product market reflects the increasing emphasis on quality assurance and regulatory compliance in diagnostic testing. As healthcare providers and laboratories strive to improve testing accuracy and reliability, the demand for quality control products, quality assurance services, and data management solutions is expected to rise. This growth is further supported by technological advancements in diagnostic testing and the increasing adoption of automated laboratory systems. Overall, the Global IVD Quality Control Product Market is poised for continued expansion, driven by the growing need for precise and reliable diagnostic tests in the healthcare industry.
| Report Metric | Details |
| Report Name | In Vitro Diagnostics (IVD) Quality Control Product Market |
| Accounted market size in 2024 | US$ 815.3 million |
| Forecasted market size in 2030 | US$ 918.2 million |
| CAGR | 2.0 |
| Base Year | 2024 |
| Forecasted years | 2025 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Bio-Rad Laboratories, Thermo Fisher Scientific, Randox Laboratories, Roche Diagnostics, Abbott Diagnostics, Helena Laboratories, Seracare Life Sciences, Technopath Clinical Diagnostics, Sun Diagnostics, Zeptometrix Corporation, ISOLAB, Sysmex Corporation, Fortress Diagnostics, Meril Life Sciences, Multiplicom, Future Diagnostics Solutions, Surmodics |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
