What is Global In Vitro Diagnostic Medical Devices and Equipment Market?
The Global In Vitro Diagnostic (IVD) Medical Devices and Equipment Market is a crucial segment of the healthcare industry that focuses on devices and tools used for diagnosing diseases and health conditions outside the human body. These devices are essential for conducting tests on samples such as blood, urine, and tissues to detect diseases, monitor health conditions, and guide treatment decisions. The market encompasses a wide range of products, including reagents, instruments, and software systems, which are used in various healthcare settings like hospitals, clinics, and laboratories. The demand for IVD devices is driven by factors such as the increasing prevalence of chronic diseases, advancements in technology, and the growing emphasis on early and accurate diagnosis. As healthcare systems worldwide strive to improve patient outcomes and reduce costs, the role of IVD devices becomes increasingly significant. These devices not only aid in the early detection of diseases but also help in monitoring ongoing treatments, thereby playing a vital role in personalized medicine. The market is characterized by continuous innovation, with companies investing heavily in research and development to introduce more efficient and accurate diagnostic tools. As a result, the Global In Vitro Diagnostic Medical Devices and Equipment Market is poised for substantial growth, driven by the need for improved diagnostic capabilities and the rising demand for healthcare services globally.
Reagent Products, Disposable Medical Supplies, Instrument, Software System, Others in the Global In Vitro Diagnostic Medical Devices and Equipment Market:
Reagent products are a fundamental component of the Global In Vitro Diagnostic Medical Devices and Equipment Market. These are chemical substances or compounds used in diagnostic tests to detect the presence of specific substances in a sample. Reagents are crucial for the accuracy and reliability of diagnostic tests, as they react with the sample to produce measurable results. They are used in a variety of tests, including blood glucose monitoring, pregnancy tests, and infectious disease detection. The demand for high-quality reagents is increasing as healthcare providers seek more precise and reliable diagnostic solutions. Disposable medical supplies, on the other hand, are single-use products that are essential for maintaining hygiene and preventing cross-contamination during diagnostic procedures. These include items like test tubes, pipettes, and gloves, which are used once and then discarded. The use of disposable supplies is critical in ensuring the safety of both patients and healthcare providers, as they minimize the risk of infection transmission. Instruments in the IVD market refer to the machines and devices used to perform diagnostic tests. These range from simple handheld devices to complex automated systems that can process multiple samples simultaneously. Instruments are designed to enhance the efficiency and accuracy of diagnostic procedures, making them indispensable in modern healthcare settings. The software systems associated with IVD devices are equally important, as they facilitate the analysis and interpretation of test results. These systems often include data management tools that help healthcare providers track patient information and monitor trends over time. The integration of software with diagnostic instruments allows for more streamlined workflows and improved decision-making in clinical settings. Other components of the IVD market include various ancillary products and services that support the overall diagnostic process. These may include calibration tools, quality control materials, and technical support services that ensure the optimal performance of diagnostic devices. Together, these elements form a comprehensive ecosystem that supports the effective and efficient delivery of diagnostic services in healthcare settings worldwide.
Hospital, Clinic, Others in the Global In Vitro Diagnostic Medical Devices and Equipment Market:
The usage of Global In Vitro Diagnostic Medical Devices and Equipment Market in hospitals is extensive and multifaceted. Hospitals rely heavily on IVD devices for a wide range of diagnostic purposes, from routine blood tests to complex genetic analyses. These devices are integral to the hospital's ability to provide timely and accurate diagnoses, which are crucial for effective patient management. In emergency departments, rapid diagnostic tests enabled by IVD devices can be life-saving, allowing for quick decision-making in critical situations. In addition, hospitals use these devices for monitoring chronic conditions, managing infectious diseases, and guiding treatment plans. The ability to conduct a variety of tests in-house reduces the need for external laboratory services, thereby improving efficiency and reducing costs. Clinics, on the other hand, utilize IVD devices to enhance their diagnostic capabilities and provide better patient care. In a clinic setting, these devices enable healthcare providers to perform point-of-care testing, which allows for immediate results and faster treatment decisions. This is particularly beneficial in primary care settings, where timely diagnosis can significantly impact patient outcomes. Clinics often use IVD devices for routine screenings, such as cholesterol and glucose tests, as well as for more specialized tests like hormone level assessments. The portability and ease of use of many IVD devices make them ideal for clinics, where space and resources may be limited. Other healthcare settings, such as research laboratories and home healthcare environments, also benefit from the advancements in IVD technology. In research labs, these devices are used to conduct experiments and develop new diagnostic methods, contributing to the advancement of medical science. In home healthcare, portable IVD devices empower patients to monitor their health conditions independently, promoting self-management and reducing the burden on healthcare facilities. Overall, the Global In Vitro Diagnostic Medical Devices and Equipment Market plays a vital role in enhancing the quality and accessibility of healthcare across various settings, ultimately improving patient outcomes and supporting the efficient delivery of medical services.
Global In Vitro Diagnostic Medical Devices and Equipment Market Outlook:
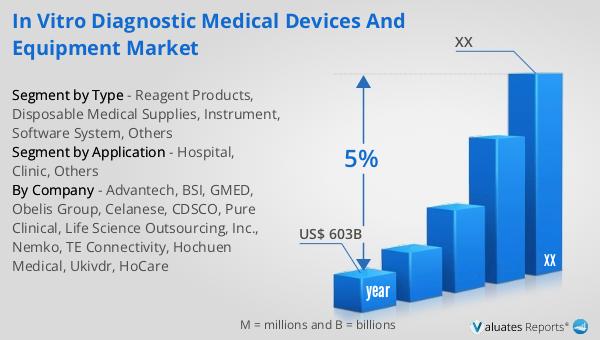
Our research indicates that the global market for medical devices is projected to reach approximately $603 billion in 2023, with an anticipated growth rate of 5% annually over the next six years. This growth trajectory underscores the increasing demand for medical devices across various healthcare sectors, driven by factors such as technological advancements, an aging population, and the rising prevalence of chronic diseases. As healthcare systems worldwide continue to evolve, the need for innovative and efficient medical devices becomes more pronounced. The projected growth rate reflects the industry's resilience and adaptability in meeting the changing needs of healthcare providers and patients alike. The expansion of the medical device market is also fueled by the growing emphasis on personalized medicine and the integration of digital technologies in healthcare. As a result, companies in this sector are investing heavily in research and development to introduce cutting-edge products that enhance diagnostic accuracy, improve patient outcomes, and streamline healthcare delivery. The market's growth is further supported by favorable regulatory environments and increased healthcare spending in emerging economies, which are driving the adoption of advanced medical technologies. Overall, the global medical device market is poised for significant growth, offering numerous opportunities for innovation and collaboration among industry stakeholders.
| Report Metric | Details |
| Report Name | In Vitro Diagnostic Medical Devices and Equipment Market |
| Accounted market size in year | US$ 603 billion |
| CAGR | 5% |
| Base Year | year |
| Segment by Type |
|
| Segment by Application |
|
| Segment by Region |
|
| By Company | GMED, Obelis Group, Celanese, CDSCO, Pure Clinical, Life Science Outsourcing, Inc., Nemko, TE Connectivity, Hochuen Medical, Ukivdr, HoCare |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
