What is Rare Disease Clinical Trial - Global Market?
Rare Disease Clinical Trials are specialized research studies aimed at developing treatments for rare diseases, which are conditions affecting a small percentage of the population. These trials are crucial because they address the unique challenges posed by rare diseases, such as limited patient populations and a lack of existing research. The global market for these trials is expanding as pharmaceutical companies and research institutions recognize the unmet medical needs and potential for innovation in this field. Conducting clinical trials for rare diseases involves navigating complex regulatory requirements and often requires collaboration between multiple stakeholders, including patients, healthcare providers, and regulatory agencies. The trials are designed to evaluate the safety and efficacy of new treatments, often involving innovative methodologies to overcome the challenges of small sample sizes. As awareness and advocacy for rare diseases grow, the demand for clinical trials in this area is expected to increase, driving advancements in medical research and offering hope to patients with rare conditions. The global market for Rare Disease Clinical Trials is a dynamic and evolving field, reflecting the broader trends in personalized medicine and the push for more targeted therapeutic approaches.
Stage I, Phase II in the Rare Disease Clinical Trial - Global Market:
Stage I and Phase II of rare disease clinical trials are critical phases in the development of new treatments. Stage I, also known as Phase I, primarily focuses on assessing the safety and tolerability of a new drug or treatment. This phase typically involves a small group of healthy volunteers or patients and aims to determine the appropriate dosage range and identify any potential side effects. In the context of rare diseases, Stage I trials can be particularly challenging due to the limited number of patients available for participation. Researchers must carefully design these trials to ensure that they gather sufficient data while minimizing risks to participants. The success of Stage I trials is crucial as it sets the foundation for subsequent phases of clinical development. Phase II trials, on the other hand, are designed to evaluate the efficacy of a treatment, as well as further assess its safety. This phase involves a larger group of patients who have the specific rare disease being studied. The primary goal of Phase II trials is to determine whether the treatment has a beneficial effect on the disease and to gather more detailed information about its safety profile. In rare disease clinical trials, Phase II can be particularly complex due to the heterogeneity of patient populations and the variability in disease progression. Researchers often employ innovative trial designs, such as adaptive trials or the use of biomarkers, to enhance the efficiency and effectiveness of Phase II studies. One of the key challenges in conducting Phase II trials for rare diseases is patient recruitment. Given the small number of individuals affected by rare diseases, finding enough participants to generate statistically significant results can be difficult. To address this, researchers may collaborate with patient advocacy groups and leverage global networks to identify and enroll eligible patients. Additionally, regulatory agencies may offer incentives, such as orphan drug designations, to encourage the development of treatments for rare diseases and facilitate the approval process. Another important aspect of Phase II trials is the selection of appropriate endpoints. In rare disease research, traditional clinical endpoints may not always be feasible or relevant due to the unique characteristics of the disease. Researchers must carefully consider alternative endpoints, such as patient-reported outcomes or surrogate markers, to accurately assess the treatment's impact. This requires a deep understanding of the disease biology and close collaboration with experts in the field. Overall, Stage I and Phase II of rare disease clinical trials are essential steps in the development of new therapies. These phases provide critical information about the safety and efficacy of treatments, guiding decisions about whether to proceed to later stages of clinical development. Despite the challenges associated with conducting trials for rare diseases, advances in technology and increased collaboration among stakeholders are helping to overcome these obstacles. As a result, the global market for rare disease clinical trials continues to grow, offering new hope for patients with rare conditions.
Autoimmunity and Inflammation, Blood System Disease, Other in the Rare Disease Clinical Trial - Global Market:
Rare Disease Clinical Trials play a significant role in advancing treatments for various medical conditions, including autoimmunity and inflammation, blood system diseases, and other rare disorders. In the area of autoimmunity and inflammation, these trials are crucial for developing therapies that target the underlying mechanisms of diseases such as lupus, rheumatoid arthritis, and multiple sclerosis. Rare disease clinical trials in this field often focus on identifying novel targets for intervention and evaluating the efficacy of new biologic agents or small molecules. Given the complexity of autoimmune diseases, these trials require a comprehensive understanding of the immune system and its dysregulation in rare conditions. Researchers work closely with immunologists and rheumatologists to design trials that address the specific needs of patients with rare autoimmune disorders. In the context of blood system diseases, rare disease clinical trials are essential for developing treatments for conditions such as hemophilia, sickle cell anemia, and thalassemia. These trials often involve gene therapy approaches or the development of novel clotting factors to address the underlying genetic defects or deficiencies. Conducting clinical trials for rare blood disorders presents unique challenges, including the need for specialized expertise in hematology and the management of potential complications related to bleeding or clotting. Researchers collaborate with hematologists and geneticists to design trials that ensure patient safety while maximizing the potential for therapeutic benefit. Beyond autoimmunity and blood system diseases, rare disease clinical trials encompass a wide range of other conditions, each with its own set of challenges and opportunities. For example, trials for rare neurological disorders, such as Huntington's disease or amyotrophic lateral sclerosis (ALS), focus on developing treatments that can slow disease progression or alleviate symptoms. These trials often involve the use of advanced imaging techniques or biomarkers to monitor disease activity and assess treatment efficacy. Similarly, rare disease trials in the field of oncology aim to develop targeted therapies for rare cancers, leveraging insights from genomics and personalized medicine to identify patients who are most likely to benefit from specific treatments. Overall, the usage of rare disease clinical trials in these areas highlights the importance of tailored approaches to drug development. Each rare disease presents unique challenges that require innovative trial designs and close collaboration among researchers, clinicians, and patients. As the global market for rare disease clinical trials continues to expand, there is growing recognition of the need for more efficient and effective strategies to bring new treatments to patients with rare conditions. This includes leveraging advances in technology, such as digital health tools and real-world data, to enhance trial design and execution. By addressing the specific needs of patients with rare diseases, these trials have the potential to transform the landscape of medical research and improve outcomes for individuals with these challenging conditions.
Rare Disease Clinical Trial - Global Market Outlook:
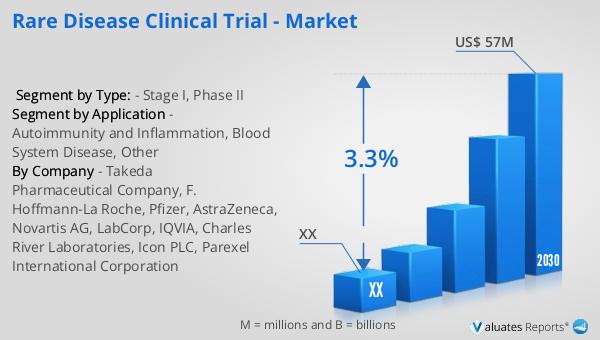
The global market for Rare Disease Clinical Trials was valued at approximately $45 million in 2023. Looking ahead, this market is projected to grow, reaching an estimated size of $57 million by 2030. This growth represents a compound annual growth rate (CAGR) of 3.3% during the forecast period from 2024 to 2030. This upward trend reflects the increasing recognition of the importance of developing treatments for rare diseases, which often lack effective therapies. The market's expansion is driven by several factors, including advancements in medical research, increased funding for rare disease initiatives, and growing awareness among healthcare professionals and patients. As more pharmaceutical companies and research institutions invest in rare disease clinical trials, the market is expected to continue its growth trajectory. This growth not only highlights the potential for innovation in the field of rare diseases but also underscores the need for continued collaboration among stakeholders to address the unique challenges associated with conducting clinical trials for these conditions. By fostering an environment that supports research and development in rare diseases, the global market for rare disease clinical trials is poised to make significant contributions to improving patient outcomes and advancing medical knowledge.
| Report Metric | Details |
| Report Name | Rare Disease Clinical Trial - Market |
| Forecasted market size in 2030 | US$ 57 million |
| CAGR | 3.3% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Takeda Pharmaceutical Company, F. Hoffmann-La Roche, Pfizer, AstraZeneca, Novartis AG, LabCorp, IQVIA, Charles River Laboratories, Icon PLC, Parexel International Corporation |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
