What is Point of Care Coagulation Testing Devices - Global Market?
Point of Care Coagulation Testing Devices are specialized medical tools designed to quickly assess the coagulation status of a patient's blood at the site of care, such as a hospital or clinic, rather than in a laboratory. These devices are crucial in managing patients who are at risk of bleeding or clotting disorders, as they provide rapid results that can guide immediate treatment decisions. The global market for these devices is expanding due to the increasing prevalence of cardiovascular diseases, the rising number of surgical procedures, and the growing demand for rapid diagnostic testing. These devices are particularly beneficial in emergency situations where time is of the essence, allowing healthcare providers to make swift and informed decisions. The market is also driven by technological advancements that have improved the accuracy and ease of use of these devices, making them more accessible to a wider range of healthcare settings. As healthcare systems worldwide continue to emphasize the importance of point-of-care testing, the demand for coagulation testing devices is expected to grow, offering significant opportunities for manufacturers and healthcare providers alike.
Anticoagulation Monitoring Devices, Platelet Function Monitoring Devices, Viscoelastic Coagulation Monitoring Devices, Others in the Point of Care Coagulation Testing Devices - Global Market:
Anticoagulation Monitoring Devices are a subset of Point of Care Coagulation Testing Devices that focus specifically on monitoring patients who are on anticoagulant therapy. These devices are essential for patients taking medications like warfarin, which require regular monitoring to ensure that blood clotting levels remain within a therapeutic range. By providing immediate feedback on a patient's coagulation status, these devices help prevent complications such as excessive bleeding or thrombosis. Platelet Function Monitoring Devices, on the other hand, assess the functionality of platelets, which are critical components of blood clotting. These devices are particularly useful for patients undergoing antiplatelet therapy, such as those with coronary artery disease, as they help determine the effectiveness of the treatment and guide necessary adjustments. Viscoelastic Coagulation Monitoring Devices offer a more comprehensive analysis of the coagulation process by measuring the viscoelastic properties of blood as it clots. This type of testing provides detailed information about the entire coagulation cascade, making it invaluable in complex clinical scenarios such as trauma or major surgery. Other devices in the Point of Care Coagulation Testing market include those that measure specific coagulation factors or markers, providing targeted insights into particular aspects of the coagulation process. These devices are continually evolving, with advancements in technology leading to more accurate, user-friendly, and cost-effective solutions. The integration of digital technologies, such as connectivity features that allow for data sharing and remote monitoring, is also enhancing the utility of these devices. As the global healthcare landscape shifts towards personalized medicine and precision healthcare, the role of these devices in tailoring treatment plans to individual patient needs is becoming increasingly important. The market for these devices is characterized by a diverse range of products, each designed to meet specific clinical needs, and is supported by a robust pipeline of innovations aimed at improving patient outcomes.
Hospitals and Clinics, Homecare, Others in the Point of Care Coagulation Testing Devices - Global Market:
Point of Care Coagulation Testing Devices are utilized in various healthcare settings, each with unique requirements and benefits. In hospitals and clinics, these devices are indispensable tools for managing patients with coagulation disorders or those undergoing procedures that carry a risk of bleeding. They enable healthcare providers to quickly assess a patient's coagulation status and make informed decisions about treatment, such as adjusting anticoagulant dosages or administering clotting factors. This rapid testing capability is particularly valuable in emergency departments, intensive care units, and surgical settings, where timely intervention can significantly impact patient outcomes. In homecare settings, Point of Care Coagulation Testing Devices empower patients to take an active role in managing their health. Patients on long-term anticoagulation therapy can use these devices to monitor their coagulation status regularly, reducing the need for frequent hospital visits and allowing for more flexible and personalized care. This not only enhances patient convenience but also improves adherence to treatment regimens, ultimately leading to better health outcomes. Other settings where these devices are used include nursing homes, rehabilitation centers, and even in some cases, remote or resource-limited areas where access to laboratory testing is challenging. In these environments, Point of Care Coagulation Testing Devices provide a critical solution for monitoring and managing coagulation disorders, ensuring that patients receive timely and appropriate care. The versatility and portability of these devices make them well-suited for a wide range of applications, supporting the broader trend towards decentralized and patient-centered healthcare. As healthcare systems continue to evolve, the role of Point of Care Coagulation Testing Devices in improving access to care and enhancing the quality of patient management is likely to expand further.
Point of Care Coagulation Testing Devices - Global Market Outlook:
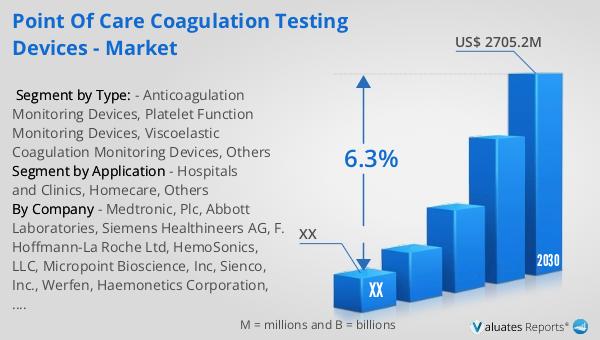
The global market for Point of Care Coagulation Testing Devices was valued at approximately $1,763 million in 2023. It is projected to grow to a revised size of around $2,705.2 million by 2030, reflecting a compound annual growth rate (CAGR) of 6.3% over the forecast period from 2024 to 2030. This growth is driven by several factors, including the increasing prevalence of coagulation disorders, the rising demand for rapid diagnostic testing, and advancements in technology that have improved the accuracy and usability of these devices. In North America, the market for Point of Care Coagulation Testing Devices was valued at a significant amount in 2023 and is expected to continue its growth trajectory through 2030. The region's market dynamics are influenced by a well-established healthcare infrastructure, high adoption rates of advanced medical technologies, and a growing focus on personalized medicine. As healthcare providers and patients alike recognize the benefits of point-of-care testing, the demand for coagulation testing devices is anticipated to rise, offering substantial opportunities for market participants. The ongoing shift towards decentralized healthcare and the increasing emphasis on patient-centered care are also expected to contribute to the market's expansion, as these trends align with the capabilities and advantages of Point of Care Coagulation Testing Devices.
| Report Metric | Details |
| Report Name | Point of Care Coagulation Testing Devices - Market |
| Forecasted market size in 2030 | US$ 2705.2 million |
| CAGR | 6.3% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Medtronic, Plc, Abbott Laboratories, Siemens Healthineers AG, F. Hoffmann-La Roche Ltd, HemoSonics, LLC, Micropoint Bioscience, Inc, Sienco, Inc., Werfen, Haemonetics Corporation, Koninklijke Philips N.V. |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
