What is Balloon Catheter Spine Augmentation System - Global Market?
Balloon Catheter Spine Augmentation Systems are specialized medical devices used in minimally invasive procedures to treat vertebral compression fractures, often caused by osteoporosis or trauma. These systems involve the insertion of a balloon catheter into the fractured vertebra, which is then inflated to create a cavity. This cavity is subsequently filled with bone cement to stabilize the fracture and restore the vertebra's height. The global market for these systems is driven by the increasing prevalence of osteoporosis, a growing elderly population, and advancements in medical technology. As awareness of minimally invasive procedures grows, so does the demand for these systems. They offer significant benefits over traditional surgical methods, including reduced recovery time, less postoperative pain, and lower risk of complications. The market is also influenced by the rising healthcare expenditure and the increasing adoption of advanced medical devices in emerging economies. As a result, the Balloon Catheter Spine Augmentation System market is poised for substantial growth, with manufacturers focusing on innovation and expanding their product portfolios to meet the diverse needs of healthcare providers and patients worldwide.
Small Balloon (2-5mm), Ordinary Balloon (5-12mm), Large Balloon (>12mm) in the Balloon Catheter Spine Augmentation System - Global Market:
Balloon Catheter Spine Augmentation Systems come in various sizes, each designed to address specific clinical needs. Small balloons, ranging from 2-5mm, are typically used in delicate procedures where precision is paramount. These smaller balloons are ideal for treating fractures in smaller vertebrae or in patients with particularly fragile bones. Their compact size allows for careful manipulation and placement, minimizing the risk of further damage to the vertebrae. Ordinary balloons, measuring between 5-12mm, are the most commonly used in spine augmentation procedures. They offer a balance between size and functionality, making them suitable for a wide range of vertebral fractures. These balloons are versatile and can be used in various clinical scenarios, providing adequate space for bone cement injection while ensuring the vertebra's structural integrity is restored. Large balloons, greater than 12mm, are employed in cases where significant vertebral height restoration is required. These are often used in patients with severe compression fractures or in larger vertebrae. The larger size allows for the creation of a substantial cavity, which can accommodate more bone cement, thereby providing enhanced stability and support to the affected vertebra. The choice of balloon size is crucial and depends on several factors, including the patient's anatomy, the severity of the fracture, and the specific clinical objectives of the procedure. Physicians must carefully assess these factors to select the appropriate balloon size, ensuring optimal outcomes for the patient. The global market for these balloon sizes is influenced by the prevalence of vertebral fractures, advancements in balloon catheter technology, and the growing demand for minimally invasive spine procedures. Manufacturers are continually innovating to develop balloons that offer improved performance, ease of use, and safety. This includes the development of balloons with enhanced materials that provide better inflation control and durability. Additionally, the integration of imaging technologies with balloon catheter systems is becoming increasingly common, allowing for more precise placement and inflation of the balloons. This technological advancement is expected to drive the adoption of balloon catheter spine augmentation systems across various healthcare settings. As the market continues to evolve, the demand for different balloon sizes will likely grow, driven by the need for personalized treatment options and improved patient outcomes.
Percutaneous Kyphoplasty, Percutaneous Vertebroplasty in the Balloon Catheter Spine Augmentation System - Global Market:
Balloon Catheter Spine Augmentation Systems are primarily used in two key procedures: Percutaneous Kyphoplasty and Percutaneous Vertebroplasty. Percutaneous Kyphoplasty involves the insertion of a balloon catheter into the fractured vertebra. Once in place, the balloon is inflated to create a cavity within the bone. This cavity is then filled with bone cement, which hardens to stabilize the fracture and restore the vertebra's height. This procedure is particularly beneficial for patients with severe vertebral compression fractures, as it not only stabilizes the fracture but also helps correct spinal deformities caused by the collapse of the vertebra. The use of balloon catheters in this procedure allows for controlled inflation and precise cavity creation, minimizing the risk of cement leakage and other complications. Percutaneous Vertebroplasty, on the other hand, involves the direct injection of bone cement into the fractured vertebra without the use of a balloon. While this procedure is effective in stabilizing the fracture, it does not offer the same level of height restoration as kyphoplasty. However, it is a valuable option for patients with less severe fractures or those who may not be suitable candidates for kyphoplasty. The choice between these two procedures depends on several factors, including the severity of the fracture, the patient's overall health, and the specific clinical goals. Balloon Catheter Spine Augmentation Systems play a crucial role in both procedures, providing the tools necessary for precise and effective treatment. The global market for these systems is driven by the increasing prevalence of vertebral compression fractures, advancements in medical technology, and the growing demand for minimally invasive procedures. As healthcare providers continue to seek out innovative solutions for treating spinal fractures, the use of balloon catheter systems in kyphoplasty and vertebroplasty is expected to rise. This growth is further supported by ongoing research and development efforts aimed at improving the safety, efficacy, and ease of use of these systems. As a result, Balloon Catheter Spine Augmentation Systems are poised to become an integral part of modern spinal fracture management, offering patients a less invasive and more effective treatment option.
Balloon Catheter Spine Augmentation System - Global Market Outlook:
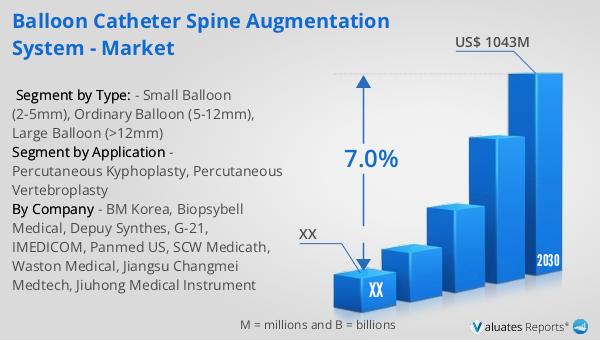
In 2023, the global market for Balloon Catheter Spine Augmentation Systems was valued at approximately $650 million. Looking ahead, this market is projected to expand significantly, reaching an estimated size of $1,043 million by 2030. This growth trajectory represents a compound annual growth rate (CAGR) of 7.0% over the forecast period from 2024 to 2030. The North American segment of this market also shows promising potential. Although specific figures for 2023 and 2030 are not provided, the region is expected to experience a steady growth rate throughout the forecast period. This growth can be attributed to several factors, including the increasing prevalence of osteoporosis and vertebral compression fractures, advancements in medical technology, and a growing preference for minimally invasive procedures. Additionally, the rising healthcare expenditure and the adoption of advanced medical devices in North America are likely to contribute to the market's expansion. As manufacturers continue to innovate and expand their product offerings, the Balloon Catheter Spine Augmentation System market is poised for substantial growth, providing healthcare providers with the tools they need to deliver effective and efficient treatment for spinal fractures.
| Report Metric | Details |
| Report Name | Balloon Catheter Spine Augmentation System - Market |
| Forecasted market size in 2030 | US$ 1043 million |
| CAGR | 7.0% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | BM Korea, Biopsybell Medical, Depuy Synthes, G-21, IMEDICOM, Panmed US, SCW Medicath, Waston Medical, Jiangsu Changmei Medtech, Jiuhong Medical Instrument |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
