What is Global Viral Clearance Service Market?
The Global Viral Clearance Service Market is a specialized sector within the broader biopharmaceutical and biotechnology industries. This market focuses on ensuring that biological products, such as vaccines, monoclonal antibodies, and gene therapies, are free from viral contaminants. Viral clearance is a critical step in the production of these products, as it ensures their safety and efficacy for human use. The process involves a series of rigorous testing and validation procedures to detect and eliminate any potential viral presence. These services are essential for compliance with regulatory standards set by health authorities like the FDA and EMA. Companies offering viral clearance services employ advanced technologies and methodologies, including viral inactivation, viral removal, and viral detection assays. The demand for these services is driven by the increasing complexity of biologics and the growing emphasis on patient safety. As the biopharmaceutical industry continues to expand, the need for reliable and efficient viral clearance services is expected to rise, making this market a crucial component of the global healthcare ecosystem.
Basic Service, Enhanced Service, Full Service, Turnkey Service in the Global Viral Clearance Service Market:
In the Global Viral Clearance Service Market, various service levels cater to different needs and requirements. Basic Service typically includes fundamental viral detection and inactivation processes. This level of service is suitable for early-stage research and development projects where preliminary viral safety assessments are needed. Enhanced Service builds upon the Basic Service by incorporating more comprehensive testing and validation procedures. This may include additional viral removal steps and more sophisticated detection assays, making it ideal for later-stage development and clinical trials. Full Service offers a complete suite of viral clearance solutions, encompassing all aspects of viral detection, inactivation, and removal. This level of service is designed for products nearing commercialization, ensuring they meet the stringent regulatory requirements for market approval. Turnkey Service is the most comprehensive offering, providing end-to-end viral clearance solutions. This includes everything from initial risk assessments and process development to final validation and regulatory submission support. Turnkey Service is particularly beneficial for companies looking to outsource their entire viral clearance process, allowing them to focus on core competencies while ensuring their products are safe and compliant. Each of these service levels plays a vital role in the biopharmaceutical development pipeline, addressing specific needs at different stages of product development.
Research Institution, Pharmaceutical Industry, Others in the Global Viral Clearance Service Market:
The Global Viral Clearance Service Market finds extensive usage across various sectors, including Research Institutions, the Pharmaceutical Industry, and others. In Research Institutions, viral clearance services are crucial for ensuring the safety of biological materials used in basic and applied research. These services help researchers identify and eliminate viral contaminants, thereby maintaining the integrity of their experiments and ensuring reliable results. In the Pharmaceutical Industry, viral clearance is a critical component of the drug development process. Pharmaceutical companies rely on these services to ensure that their biologic products, such as vaccines and therapeutic proteins, are free from viral contaminants. This is essential for meeting regulatory requirements and ensuring patient safety. Other sectors that benefit from viral clearance services include contract research organizations (CROs), biotechnology firms, and academic institutions. These entities often collaborate with viral clearance service providers to ensure the safety and efficacy of their biological products. Overall, the usage of viral clearance services is integral to the development and commercialization of safe and effective biologics, making it a vital component of the global healthcare landscape.
Global Viral Clearance Service Market Outlook:
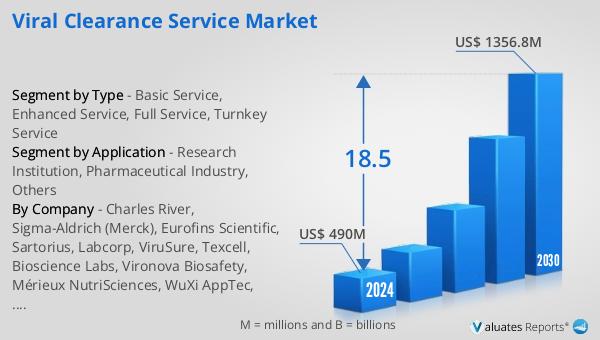
The global market for Viral Clearance Service was estimated to be worth US$ 408.1 million in 2023 and is forecast to a readjusted size of US$ 1356.8 million by 2030 with a CAGR of 18.5% during the forecast period 2024-2030. North America is the largest Viral Clearance Service market with about 51% market share. Europe is follower accounting for about 23% market share. Top 3 companies occupied about 39% market share.
| Report Metric | Details |
| Report Name | Viral Clearance Service Market |
| Forecasted market size in 2030 | US$ 1356.8 million |
| CAGR | 18.5% |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Charles River, Sigma-Aldrich (Merck), Eurofins Scientific, Sartorius, Labcorp, ViruSure, Texcell, Bioscience Labs, Vironova Biosafety, Mérieux NutriSciences, WuXi AppTec, Syngene, Labor Dr. Merk (Boehringer Ingelheim), Sino Biological |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
