What is Global RSV Vaccine Market?
The Global RSV Vaccine Market refers to the worldwide industry focused on the development, production, and distribution of vaccines for Respiratory Syncytial Virus (RSV). RSV is a common respiratory virus that can cause severe infections, particularly in infants, young children, and the elderly. The market encompasses various stakeholders, including pharmaceutical companies, research institutions, and healthcare providers, all working towards creating effective vaccines to combat RSV. The demand for RSV vaccines is driven by the high incidence of RSV infections and the significant health risks associated with the virus, especially in vulnerable populations. As a result, the market is characterized by ongoing research and development activities, clinical trials, and regulatory approvals aimed at bringing new and improved vaccines to the market. The ultimate goal is to reduce the global burden of RSV-related illnesses and deaths through widespread vaccination programs.
Clinical I, Clinical II, Clinical III, Preclinical, Being Developed in the Global RSV Vaccine Market:
The development of vaccines in the Global RSV Vaccine Market involves several stages, including Preclinical, Clinical I, Clinical II, and Clinical III phases, as well as ongoing development efforts. The Preclinical stage is the initial phase where researchers conduct laboratory and animal studies to evaluate the safety and efficacy of potential vaccine candidates. This stage is crucial for identifying promising candidates that can move forward to human trials. Once a vaccine candidate shows potential in preclinical studies, it enters the Clinical I phase. In this phase, the vaccine is tested on a small group of healthy volunteers to assess its safety, dosage, and potential side effects. The primary goal is to ensure that the vaccine is safe for human use before proceeding to larger trials. If the vaccine passes the Clinical I phase, it advances to the Clinical II phase, where it is tested on a larger group of participants, including those from the target population. This phase aims to further evaluate the vaccine's safety, immunogenicity (the ability to provoke an immune response), and optimal dosing regimen. Researchers also gather preliminary data on the vaccine's effectiveness in preventing RSV infections. The Clinical III phase is the final and most extensive stage of clinical testing. It involves thousands of participants and is designed to confirm the vaccine's safety and efficacy on a larger scale. This phase provides the critical data needed for regulatory approval. Successful completion of Clinical III trials can lead to the submission of a Biologics License Application (BLA) or Marketing Authorization Application (MAA) to regulatory agencies such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). Once approved, the vaccine can be manufactured and distributed for public use. However, the development process doesn't end with regulatory approval. Post-marketing surveillance and ongoing research are essential to monitor the vaccine's long-term safety and effectiveness. Additionally, new vaccine candidates and formulations continue to be developed to address emerging strains of RSV and improve overall vaccine performance. This continuous cycle of research, development, and monitoring ensures that the Global RSV Vaccine Market remains dynamic and responsive to the evolving needs of public health.
Elderly Vaccine, Infant Vaccine, Others in the Global RSV Vaccine Market:
The usage of vaccines in the Global RSV Vaccine Market is particularly significant in three key areas: Elderly Vaccine, Infant Vaccine, and Others. For the elderly population, RSV can lead to severe respiratory illnesses, including pneumonia and bronchitis, which can be life-threatening. Vaccination in this age group aims to reduce the incidence and severity of RSV infections, thereby decreasing hospitalizations and healthcare costs. Elderly individuals often have weakened immune systems, making them more susceptible to infections. Therefore, an effective RSV vaccine can play a crucial role in protecting this vulnerable population and improving their quality of life. In the case of infants, RSV is a leading cause of respiratory infections, including bronchiolitis and pneumonia, which can result in significant morbidity and mortality. Vaccinating infants against RSV is essential to prevent these severe outcomes and reduce the burden on healthcare systems. Infant vaccines are typically administered in multiple doses to ensure robust and long-lasting immunity. The development of safe and effective RSV vaccines for infants is a top priority in the Global RSV Vaccine Market, given the high risk and potential complications associated with RSV infections in this age group. The "Others" category includes various populations and settings where RSV vaccination can be beneficial. This may include pregnant women, who can receive the vaccine to protect their newborns through passive immunity. Healthcare workers and caregivers who are in close contact with high-risk populations, such as the elderly and infants, may also benefit from vaccination to prevent the spread of RSV. Additionally, individuals with underlying health conditions, such as chronic respiratory diseases or compromised immune systems, may be prioritized for RSV vaccination to reduce their risk of severe infections. The Global RSV Vaccine Market is thus focused on developing targeted vaccination strategies to address the specific needs of these diverse populations. By doing so, the market aims to achieve comprehensive protection against RSV and mitigate its impact on public health.
Global RSV Vaccine Market Outlook:
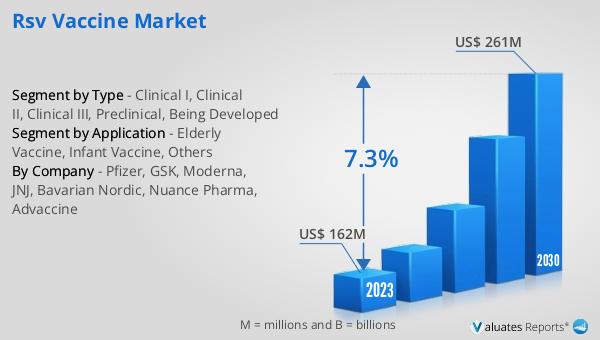
The global RSV Vaccine market was valued at US$ 162 million in 2023 and is anticipated to reach US$ 261 million by 2030, witnessing a CAGR of 7.3% during the forecast period 2024-2030. The global pharmaceutical market is 1475 billion USD in 2022, growing at a CAGR of 5% during the next six years. In comparison, the chemical drug market is estimated to increase from 1005 billion in 2018 to 1094 billion U.S. dollars in 2022. This data highlights the significant growth potential of the RSV Vaccine market within the broader pharmaceutical industry. The increasing focus on vaccine development, coupled with the rising awareness of RSV's impact on public health, is driving investments and advancements in this market. As pharmaceutical companies continue to innovate and bring new vaccines to market, the RSV Vaccine market is expected to play a crucial role in addressing the global burden of respiratory infections. The projected growth in market value underscores the importance of ongoing research and development efforts to create effective and accessible vaccines for populations at risk. By aligning with the broader trends in the pharmaceutical and chemical drug markets, the RSV Vaccine market is poised to make significant contributions to global health outcomes.
| Report Metric | Details |
| Report Name | RSV Vaccine Market |
| Accounted market size in 2023 | US$ 162 million |
| Forecasted market size in 2030 | US$ 261 million |
| CAGR | 7.3% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Pfizer, GSK, Moderna, JNJ, Bavarian Nordic, Nuance Pharma, Advaccine |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
