What is Global Non-invasive Helicobacter Pylori Diagnostic Market?
The Global Non-invasive Helicobacter Pylori Diagnostic Market refers to the worldwide industry focused on the development, production, and distribution of diagnostic tools that do not require invasive procedures to detect Helicobacter pylori (H. pylori) infections. H. pylori is a type of bacteria that infects the stomach lining and is a common cause of ulcers and other gastrointestinal issues. Non-invasive diagnostic methods include breath tests, stool antigen tests, and blood antibody tests, which are preferred over invasive techniques like endoscopy and biopsy due to their simplicity, safety, and cost-effectiveness. These non-invasive methods are crucial for early detection and management of H. pylori infections, which can lead to more serious conditions if left untreated. The market for these diagnostics is growing as awareness of H. pylori-related health issues increases and as healthcare providers seek more efficient and patient-friendly diagnostic options.
Invasive Techniques, Non-invasive Techniques in the Global Non-invasive Helicobacter Pylori Diagnostic Market:
Invasive techniques for diagnosing Helicobacter pylori involve procedures that require entering the body, typically through endoscopy or biopsy. During an endoscopy, a flexible tube with a camera is inserted through the mouth into the stomach to visually inspect the stomach lining and collect tissue samples. These samples are then tested for the presence of H. pylori bacteria. While invasive techniques are highly accurate and can provide additional information about the condition of the stomach lining, they are often uncomfortable for patients, require sedation, and carry risks such as bleeding or infection. On the other hand, non-invasive techniques offer a safer and more convenient alternative for diagnosing H. pylori infections. The most common non-invasive methods include the urea breath test, stool antigen test, and serological tests. The urea breath test involves the patient ingesting a urea solution labeled with a special carbon isotope. If H. pylori is present, the bacteria will break down the urea, releasing carbon dioxide that can be detected in the patient's breath. This test is highly accurate and provides quick results. The stool antigen test detects H. pylori antigens in a patient's fecal sample, offering a reliable and non-invasive diagnostic option. Serological tests, which detect antibodies against H. pylori in the blood, are less commonly used due to their lower accuracy compared to breath and stool tests. The global market for non-invasive H. pylori diagnostics is driven by the increasing prevalence of H. pylori infections, the growing awareness of the importance of early detection, and the demand for safer and more patient-friendly diagnostic methods. Non-invasive techniques are particularly valuable in settings where invasive procedures are not feasible or where patient compliance is a concern. They are also useful for monitoring the effectiveness of treatment, as they can be repeated without causing discomfort or risk to the patient. As healthcare systems worldwide continue to prioritize patient safety and comfort, the adoption of non-invasive diagnostic methods for H. pylori is expected to rise, further driving the growth of this market.
Hospitals and Clinics, Diagnostic Laboratories in the Global Non-invasive Helicobacter Pylori Diagnostic Market:
The usage of non-invasive Helicobacter pylori diagnostic methods in hospitals and clinics is becoming increasingly prevalent due to their convenience, safety, and efficiency. In these settings, healthcare providers often prefer non-invasive tests such as the urea breath test and stool antigen test for initial diagnosis and follow-up assessments. These tests can be easily administered without the need for specialized equipment or extensive patient preparation, making them ideal for busy clinical environments. The urea breath test, for example, can be completed in a matter of minutes and provides accurate results that help clinicians make timely decisions about treatment. Similarly, the stool antigen test is a simple and reliable method that can be used to confirm the presence of H. pylori and monitor the success of eradication therapy. Diagnostic laboratories also play a crucial role in the global non-invasive Helicobacter pylori diagnostic market. These laboratories are equipped with advanced technologies and trained personnel to perform a wide range of diagnostic tests, including non-invasive methods for detecting H. pylori. In diagnostic laboratories, the urea breath test and stool antigen test are commonly used due to their high sensitivity and specificity. These tests are particularly valuable in large-scale screening programs and epidemiological studies, where accurate and efficient detection of H. pylori is essential. The ability to process multiple samples simultaneously and provide rapid results makes non-invasive tests an attractive option for diagnostic laboratories. Moreover, the use of non-invasive diagnostic methods in both hospitals and clinics, as well as diagnostic laboratories, aligns with the broader trend towards patient-centered care. By minimizing discomfort and reducing the risks associated with invasive procedures, non-invasive tests enhance the overall patient experience and improve compliance with diagnostic and treatment protocols. This is especially important in the management of H. pylori infections, where timely and accurate diagnosis is critical for preventing complications such as peptic ulcers and gastric cancer. As healthcare providers and diagnostic laboratories continue to adopt non-invasive methods, the global market for these diagnostics is expected to grow, driven by the increasing demand for safe, efficient, and patient-friendly diagnostic solutions.
Global Non-invasive Helicobacter Pylori Diagnostic Market Outlook:
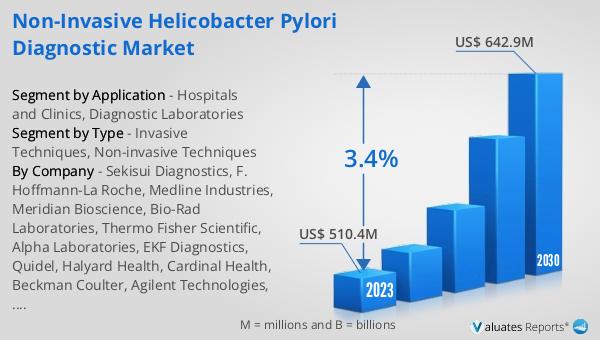
The global Non-invasive Helicobacter Pylori Diagnostic market was valued at US$ 510.4 million in 2023 and is projected to reach US$ 642.9 million by 2030, reflecting a compound annual growth rate (CAGR) of 3.4% during the forecast period from 2024 to 2030. In comparison, the global pharmaceutical market was valued at 1475 billion USD in 2022 and is expected to grow at a CAGR of 5% over the next six years. Meanwhile, the chemical drug market is estimated to have increased from 1005 billion USD in 2018 to 1094 billion USD in 2022. These figures highlight the significant growth potential of the non-invasive H. pylori diagnostic market within the broader context of the pharmaceutical and chemical drug markets. The increasing prevalence of H. pylori infections, coupled with the growing demand for safer and more patient-friendly diagnostic methods, is driving the expansion of this market. As healthcare providers and patients alike seek more convenient and less invasive options for diagnosing H. pylori, the adoption of non-invasive diagnostic methods is expected to rise, further fueling market growth.
| Report Metric | Details |
| Report Name | Non-invasive Helicobacter Pylori Diagnostic Market |
| Accounted market size in 2023 | US$ 510.4 million |
| Forecasted market size in 2030 | US$ 642.9 million |
| CAGR | 3.4% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Sekisui Diagnostics, F. Hoffmann-La Roche, Medline Industries, Meridian Bioscience, Bio-Rad Laboratories, Thermo Fisher Scientific, Alpha Laboratories, EKF Diagnostics, Quidel, Halyard Health, Cardinal Health, Beckman Coulter, Agilent Technologies, Coris BioConcept |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
