What is Global Clinical Research & Development Solution Market?
The Global Clinical Research & Development Solution Market is a rapidly evolving sector that plays a crucial role in the healthcare industry. This market encompasses a wide range of services and solutions aimed at facilitating the development and approval of new drugs and therapies. It includes activities such as clinical trials, regulatory compliance, data management, and patient recruitment, among others. The primary goal of this market is to ensure that new medical treatments are safe, effective, and meet regulatory standards before they are made available to the public. Companies operating in this market provide essential support to pharmaceutical and biotechnological firms, helping them navigate the complex and often lengthy process of bringing new drugs to market. With advancements in technology and increasing demand for innovative treatments, the Global Clinical Research & Development Solution Market is expected to continue its growth trajectory, offering new opportunities for stakeholders involved in drug development and clinical research.
Early Phase Services (Phase I to IIa), Study Design, Planning & Execution, Decentralized Clinical Trials, Others in the Global Clinical Research & Development Solution Market:
Early Phase Services, encompassing Phase I to IIa, are critical components of the Global Clinical Research & Development Solution Market. These phases are the initial steps in the clinical trial process, where the safety, tolerability, pharmacokinetics, and pharmacodynamics of a new drug are evaluated. During Phase I, the drug is tested on a small group of healthy volunteers to assess its safety and determine the appropriate dosage range. Phase IIa involves a slightly larger group of patients who have the condition the drug is intended to treat, focusing on the drug's efficacy and side effects. Study Design, Planning & Execution are integral to the success of clinical trials. This involves developing a comprehensive plan that outlines the objectives, methodology, and statistical considerations of the study. Effective planning ensures that the trial is conducted efficiently, ethically, and in compliance with regulatory requirements. Execution involves the actual implementation of the study, including patient recruitment, data collection, and monitoring. Decentralized Clinical Trials (DCTs) are an emerging trend in the Global Clinical Research & Development Solution Market. DCTs leverage digital technologies to conduct clinical trials remotely, reducing the need for patients to visit clinical sites. This approach enhances patient convenience, increases participation rates, and accelerates the trial process. DCTs utilize telemedicine, mobile health apps, and wearable devices to collect data and monitor patients in real-time. Other services in this market include regulatory consulting, data management, biostatistics, and medical writing. Regulatory consulting helps companies navigate the complex regulatory landscape, ensuring that their clinical trials comply with local and international guidelines. Data management involves the collection, storage, and analysis of clinical trial data, ensuring its accuracy and integrity. Biostatistics plays a crucial role in designing studies, analyzing data, and interpreting results. Medical writing involves the preparation of clinical trial documents, including study protocols, informed consent forms, and regulatory submissions. These services collectively contribute to the efficient and successful execution of clinical trials, ultimately leading to the development of new and innovative medical treatments.
Pharmaceutical & Biotechnological Companies, Academic & Research Institutes, Others in the Global Clinical Research & Development Solution Market:
The Global Clinical Research & Development Solution Market is extensively utilized by Pharmaceutical & Biotechnological Companies, Academic & Research Institutes, and other stakeholders. Pharmaceutical and biotechnological companies are the primary users of these solutions, as they are responsible for developing new drugs and therapies. These companies rely on clinical research and development services to conduct clinical trials, manage data, and ensure regulatory compliance. By outsourcing these activities to specialized service providers, pharmaceutical and biotechnological companies can focus on their core competencies, such as drug discovery and development. Clinical research and development solutions help these companies accelerate the drug development process, reduce costs, and improve the chances of regulatory approval. Academic and research institutes also play a significant role in the Global Clinical Research & Development Solution Market. These institutions conduct independent research to advance scientific knowledge and develop new medical treatments. They often collaborate with pharmaceutical and biotechnological companies to conduct clinical trials and validate the efficacy and safety of new drugs. Academic and research institutes benefit from clinical research and development solutions by gaining access to advanced technologies, expertise, and resources. This collaboration fosters innovation and contributes to the overall advancement of medical science. Other stakeholders in this market include contract research organizations (CROs), regulatory authorities, and healthcare providers. CROs are specialized service providers that offer a wide range of clinical research and development services to pharmaceutical and biotechnological companies. They play a crucial role in managing and executing clinical trials, ensuring that they are conducted efficiently and in compliance with regulatory requirements. Regulatory authorities are responsible for overseeing the approval and monitoring of new drugs and therapies. They rely on clinical research and development solutions to evaluate the safety and efficacy of new treatments and ensure that they meet regulatory standards. Healthcare providers, including hospitals and clinics, also utilize these solutions to conduct clinical trials and contribute to the development of new medical treatments. By participating in clinical trials, healthcare providers can offer their patients access to cutting-edge therapies and contribute to the advancement of medical science.
Global Clinical Research & Development Solution Market Outlook:
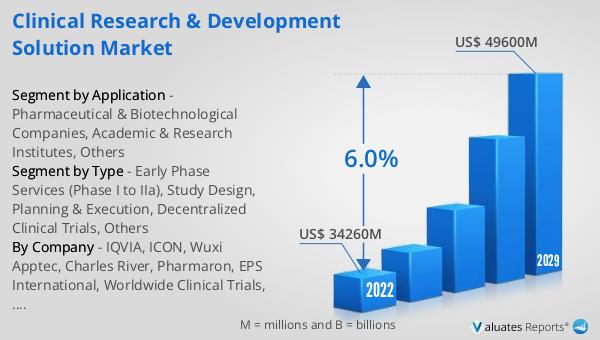
The global Clinical Research & Development Solution market was valued at US$ 34,260 million in 2023 and is anticipated to reach US$ 49,600 million by 2030, witnessing a CAGR of 6.0% during the forecast period 2024-2030. This market outlook highlights the significant growth potential of the Global Clinical Research & Development Solution Market over the next several years. The increasing demand for innovative medical treatments, coupled with advancements in technology, is expected to drive the growth of this market. Pharmaceutical and biotechnological companies are investing heavily in research and development to bring new drugs to market, and they rely on clinical research and development solutions to streamline the process. The adoption of decentralized clinical trials and other advanced methodologies is also contributing to the market's growth by enhancing the efficiency and effectiveness of clinical trials. As the market continues to expand, stakeholders, including pharmaceutical companies, academic institutions, and regulatory authorities, will benefit from the improved capabilities and resources offered by clinical research and development solutions. This growth trajectory underscores the importance of the Global Clinical Research & Development Solution Market in advancing medical science and improving patient outcomes.
| Report Metric | Details |
| Report Name | Clinical Research & Development Solution Market |
| Accounted market size in 2023 | US$ 34260 million |
| Forecasted market size in 2030 | US$ 49600 million |
| CAGR | 6.0% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | IQVIA, ICON, Wuxi Apptec, Charles River, Pharmaron, EPS International, Worldwide Clinical Trials, CMIC, Inotiv, JOINN Lab, ChemPartner, Medicilon, EVOTEC, Labcorp, Syneos Health, Medpace, Parexel |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
