What is Global Water for Injection (WFI) Market?
The Global Water for Injection (WFI) Market refers to the industry that produces and supplies Water for Injection, a type of water that meets stringent purity standards set by pharmacopeias such as the United States Pharmacopeia (USP) and the European Pharmacopoeia (EP). WFI is primarily used in the pharmaceutical and biotechnology industries for the preparation of parenteral solutions, which are administered directly into the bloodstream. This type of water is free from any contaminants, including bacteria, endotoxins, and other impurities, making it suitable for use in the production of drugs, vaccines, and other medical products. The market for WFI is driven by the increasing demand for high-quality pharmaceuticals and the growing prevalence of chronic diseases that require injectable treatments. Additionally, advancements in biotechnology and the development of new biologic drugs are further propelling the demand for WFI. The market is characterized by stringent regulatory requirements and the need for advanced production technologies to ensure the purity and safety of the water.
Bacteriostatic Water for Injection, Sterile Water for Injection in the Global Water for Injection (WFI) Market:
Bacteriostatic Water for Injection and Sterile Water for Injection are two key products within the Global Water for Injection (WFI) Market. Bacteriostatic Water for Injection contains a small amount of a bacteriostatic agent, typically 0.9% benzyl alcohol, which inhibits the growth of bacteria. This type of water is used for diluting or dissolving medications for injection, particularly when multiple doses are required from a single vial. The bacteriostatic agent helps to prevent contamination and extends the shelf life of the reconstituted medication. On the other hand, Sterile Water for Injection is free from any antimicrobial agents and is used for single-dose injections. It is used to dissolve or dilute drugs that are intended for intravenous, intramuscular, or subcutaneous administration. Both types of water must meet strict purity standards to ensure they are free from endotoxins, particulate matter, and other contaminants. The production of these waters involves multiple stages of purification, including distillation, reverse osmosis, and ultrafiltration, followed by sterilization. The demand for Bacteriostatic Water for Injection and Sterile Water for Injection is driven by the increasing prevalence of chronic diseases, the growing number of surgical procedures, and the rising demand for biologic drugs. Additionally, the expansion of the pharmaceutical and biotechnology industries, particularly in emerging markets, is contributing to the growth of the WFI market. The stringent regulatory requirements for the production and quality control of WFI products ensure that only the highest quality water is used in the preparation of injectable medications. This is crucial for patient safety and the efficacy of the medications. The market for WFI is highly competitive, with several key players investing in advanced production technologies and expanding their production capacities to meet the growing demand. The increasing focus on research and development in the pharmaceutical and biotechnology sectors is also driving the demand for high-quality WFI products. Overall, the Global Water for Injection (WFI) Market is expected to continue growing, driven by the increasing demand for injectable medications and the ongoing advancements in pharmaceutical and biotechnology research.
Research Institutes, Hospitals, Clinics, Others in the Global Water for Injection (WFI) Market:
The usage of Global Water for Injection (WFI) Market products spans across various sectors, including Research Institutes, Hospitals, Clinics, and other healthcare facilities. In Research Institutes, WFI is essential for conducting experiments and developing new drugs and vaccines. The purity of WFI ensures that there are no contaminants that could interfere with the results of the experiments or compromise the safety of the developed products. Researchers rely on WFI for cell culture, molecular biology experiments, and the preparation of reagents and buffers. In Hospitals, WFI is used for preparing injectable medications, including antibiotics, pain relievers, and other critical drugs. The high purity of WFI is crucial for patient safety, as any contaminants in the water could lead to infections or other adverse reactions. Hospitals also use WFI for reconstituting lyophilized drugs, which are commonly used in emergency and critical care settings. In Clinics, WFI is used for a variety of purposes, including the preparation of vaccines, intravenous fluids, and other injectable treatments. The use of WFI in clinics ensures that patients receive safe and effective treatments, free from any contaminants. Additionally, WFI is used in diagnostic procedures, such as the preparation of contrast agents for imaging studies. Other healthcare facilities, such as ambulatory surgical centers and long-term care facilities, also rely on WFI for the preparation of injectable medications and other treatments. The use of WFI in these settings ensures that patients receive the highest quality care, with minimal risk of infections or other complications. The demand for WFI in these various sectors is driven by the increasing prevalence of chronic diseases, the growing number of surgical procedures, and the rising demand for biologic drugs. Additionally, the expansion of healthcare infrastructure in emerging markets is contributing to the growth of the WFI market. The stringent regulatory requirements for the production and quality control of WFI products ensure that only the highest quality water is used in the preparation of injectable medications and other treatments. This is crucial for patient safety and the efficacy of the treatments. Overall, the usage of Global Water for Injection (WFI) Market products in Research Institutes, Hospitals, Clinics, and other healthcare facilities is essential for ensuring the safety and effectiveness of injectable medications and other treatments.
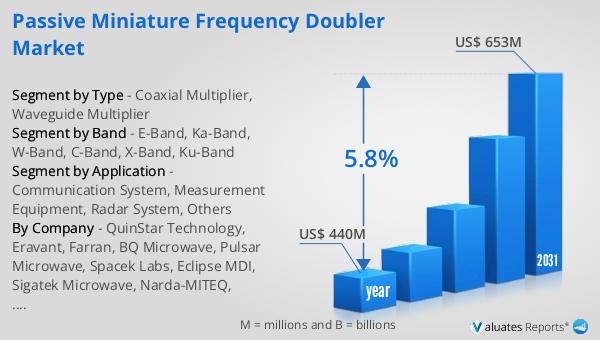
Global Water for Injection (WFI) Market Outlook:
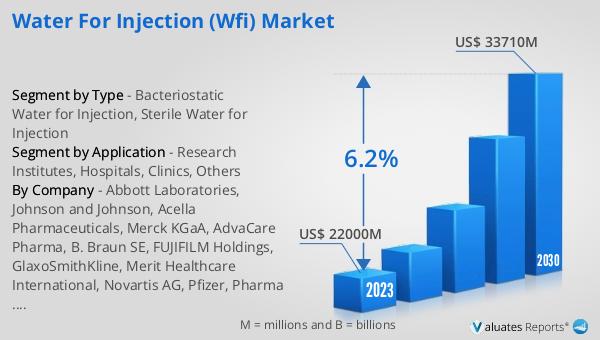
The global Water for Injection (WFI) market was valued at US$ 22,000 million in 2023 and is anticipated to reach US$ 33,710 million by 2030, witnessing a CAGR of 6.2% during the forecast period from 2024 to 2030. This significant growth reflects the increasing demand for high-quality injectable medications and the expansion of the pharmaceutical and biotechnology industries. The market's robust growth is driven by the rising prevalence of chronic diseases, the growing number of surgical procedures, and the increasing demand for biologic drugs. Additionally, advancements in biotechnology and the development of new biologic drugs are further propelling the demand for WFI. The stringent regulatory requirements for the production and quality control of WFI products ensure that only the highest quality water is used in the preparation of injectable medications. This is crucial for patient safety and the efficacy of the medications. The market is characterized by the presence of several key players who are investing in advanced production technologies and expanding their production capacities to meet the growing demand. The increasing focus on research and development in the pharmaceutical and biotechnology sectors is also driving the demand for high-quality WFI products. Overall, the global Water for Injection (WFI) market is expected to continue growing, driven by the increasing demand for injectable medications and the ongoing advancements in pharmaceutical and biotechnology research.
| Report Metric | Details |
| Report Name | Water for Injection (WFI) Market |
| Accounted market size in 2023 | US$ 22000 million |
| Forecasted market size in 2030 | US$ 33710 million |
| CAGR | 6.2% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Abbott Laboratories, Johnson and Johnson, Acella Pharmaceuticals, Merck KGaA, AdvaCare Pharma, B. Braun SE, FUJIFILM Holdings, GlaxoSmithKline, Merit Healthcare International, Novartis AG, Pfizer, Pharma Cure Laboratories, Sanofi, Zhejiang Tianrui Pharmaceutical, Zhuhai Tongyuan Pharmaceutical, Shandong Kanghua |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |