What is Global Thrombus Management Device Market?
The Global Thrombus Management Device Market refers to the worldwide industry focused on the development, production, and distribution of medical devices designed to manage and treat thrombus, which are blood clots that can obstruct blood vessels and lead to serious health complications. These devices are crucial in the treatment of conditions such as deep vein thrombosis, pulmonary embolism, and arterial thrombosis. The market encompasses a variety of devices, including neurovascular embolectomy devices, embolectomy balloon catheters, percutaneous thrombectomy devices, and catheter-guided thrombolysis devices. These devices are used in various medical settings, including hospitals and organ transplant centers, to ensure the effective removal or dissolution of blood clots, thereby restoring normal blood flow and preventing further complications. The market is driven by the increasing prevalence of thrombotic disorders, advancements in medical technology, and the growing demand for minimally invasive procedures.
Neurovascular Embolectomy Device, Embolectomy Balloon Catheter, Percutaneous Thrombectomy Device, Catheter-guided Thrombolysis Device in the Global Thrombus Management Device Market:
Neurovascular embolectomy devices are specialized tools used to remove blood clots from the neurovascular system, which includes the blood vessels in the brain and spinal cord. These devices are designed to navigate the complex and delicate neurovascular structures, ensuring precise and effective clot removal. Embolectomy balloon catheters, on the other hand, are flexible tubes with an inflatable balloon at the tip. They are inserted into the blood vessel and guided to the site of the clot. Once in position, the balloon is inflated to capture and remove the clot. Percutaneous thrombectomy devices are minimally invasive tools that use mechanical or aspiration techniques to break up and remove clots from blood vessels. These devices are inserted through a small incision in the skin and guided to the clot site, where they either mechanically disrupt the clot or use suction to remove it. Catheter-guided thrombolysis devices involve the use of a catheter to deliver clot-dissolving medication directly to the site of the thrombus. This targeted approach ensures that the medication is delivered precisely where it is needed, enhancing its effectiveness and reducing the risk of systemic side effects. Each of these devices plays a crucial role in the management of thrombotic disorders, offering different approaches to clot removal and dissolution based on the specific needs of the patient and the location of the clot.
Hospital, Organ Transplant Center in the Global Thrombus Management Device Market:
In hospitals, thrombus management devices are essential tools in the treatment of patients with thrombotic disorders. These devices are used in various departments, including emergency rooms, intensive care units, and surgical wards, to manage and treat blood clots. In emergency rooms, for example, patients presenting with symptoms of a stroke or heart attack may require immediate intervention to remove or dissolve a clot. Neurovascular embolectomy devices and catheter-guided thrombolysis devices are often used in these situations to quickly restore blood flow to the affected area and minimize damage to the brain or heart. In intensive care units, patients who are bedridden or have undergone major surgery are at increased risk of developing deep vein thrombosis or pulmonary embolism. Embolectomy balloon catheters and percutaneous thrombectomy devices are commonly used in these settings to prevent and treat these potentially life-threatening conditions. In surgical wards, thrombus management devices are used during and after surgery to ensure that blood clots do not form and cause complications. For example, during orthopedic surgeries, patients are at high risk of developing blood clots due to prolonged immobility and the nature of the surgery. Thrombus management devices are used to monitor and manage clot formation, ensuring that patients recover safely and without complications.
Global Thrombus Management Device Market Outlook:
Organ transplant centers also rely heavily on thrombus management devices to ensure the success of transplant procedures. Blood clots can pose a significant risk to both the donor organ and the recipient, potentially leading to organ failure or other serious complications. During the transplant procedure, thrombus management devices are used to ensure that the blood vessels are clear of clots and that blood flow to the transplanted organ is not obstructed. For example, during a liver transplant, the blood vessels supplying the liver must be free of clots to ensure that the organ receives adequate blood flow and functions properly. Embolectomy balloon catheters and catheter-guided thrombolysis devices are often used in these procedures to remove any clots and ensure that the blood vessels are clear. After the transplant, patients are closely monitored for signs of clot formation, and thrombus management devices are used as needed to prevent and treat any clots that may develop. This careful management of blood clots is essential to the success of the transplant and the long-term health of the patient.
| Report Metric | Details |
| Report Name | Thrombus Management Device Market |
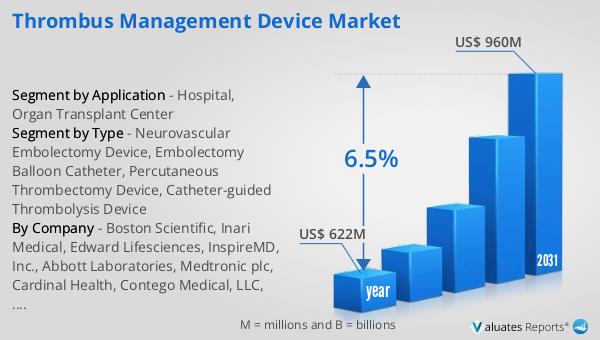
| Accounted market size in 2023 | US$ 514.5 million |
| Forecasted market size in 2030 | US$ 856.7 million |
| CAGR | 6.5% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Boston Scientific, Inari Medical, Edward Lifesciences, InspireMD, Inc., Abbott Laboratories, Medtronic plc, Cardinal Health, Contego Medical, LLC, Silk Road Medical, Inc., Edwards, Lifesciences Corporation, Lepu Medical Technology |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
